Research Articles
Strategies to Minimize False Positives in qPCR Cancer Assays: From Foundational Principles to Clinical Validation
This article provides a comprehensive guide for researchers and drug development professionals on mitigating false positive results in quantitative PCR (qPCR) assays for cancer detection and monitoring.
Troubleshooting High Ct Values in Cancer Biomarker Detection: A Strategic Guide for Robust qPCR and dPCR Results
This article provides a comprehensive guide for researchers and drug development professionals facing the challenge of high cycle threshold (Ct) values in the detection of low-abundance cancer biomarkers.
Maximizing qPCR Efficiency with Challenging RNA Samples: A Guide to Robust Gene Expression Data
Obtaining reliable quantitative PCR (qPCR) results from low-quality or inhibitor-rich RNA samples is a common challenge in biomedical research and drug development.
Optimizing qPCR for Minimal Residual Disease Detection: Protocols, Pitfalls, and Future Directions
This article provides a comprehensive guide for researchers and drug development professionals on implementing quantitative PCR (qPCR) for minimal residual disease (MRD) detection.
qPCR for Copy Number Alteration Analysis in Oral Cancer: A Comprehensive Guide for Biomarker Validation and Clinical Research
Copy number alterations (CNAs) are critical drivers of oral squamous cell carcinoma (OSCC), influencing oncogene activation, tumor suppressor silencing, and patient prognosis.
RT-qPCR for Angiogenesis Biomarkers in Tumor Tissue: A Comprehensive Guide from Biomarker Discovery to Clinical Validation
This article provides a comprehensive guide for researchers and drug development professionals on the application of Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) for analyzing angiogenesis biomarkers in tumor tissue.
Optimized RT-qPCR Protocol for Plasma miRNA Quantification: A Standardized Workflow for Reliable Biomarker Research
Circulating microRNAs in plasma have emerged as promising minimally invasive biomarkers for various diseases, from cancer to ageing-related disorders.
Advanced TaqMan Assay Protocols for Sensitive Cancer Mutation Detection
This article provides a comprehensive guide for researchers and drug development professionals on implementing TaqMan assay protocols for somatic mutation detection in cancer research.
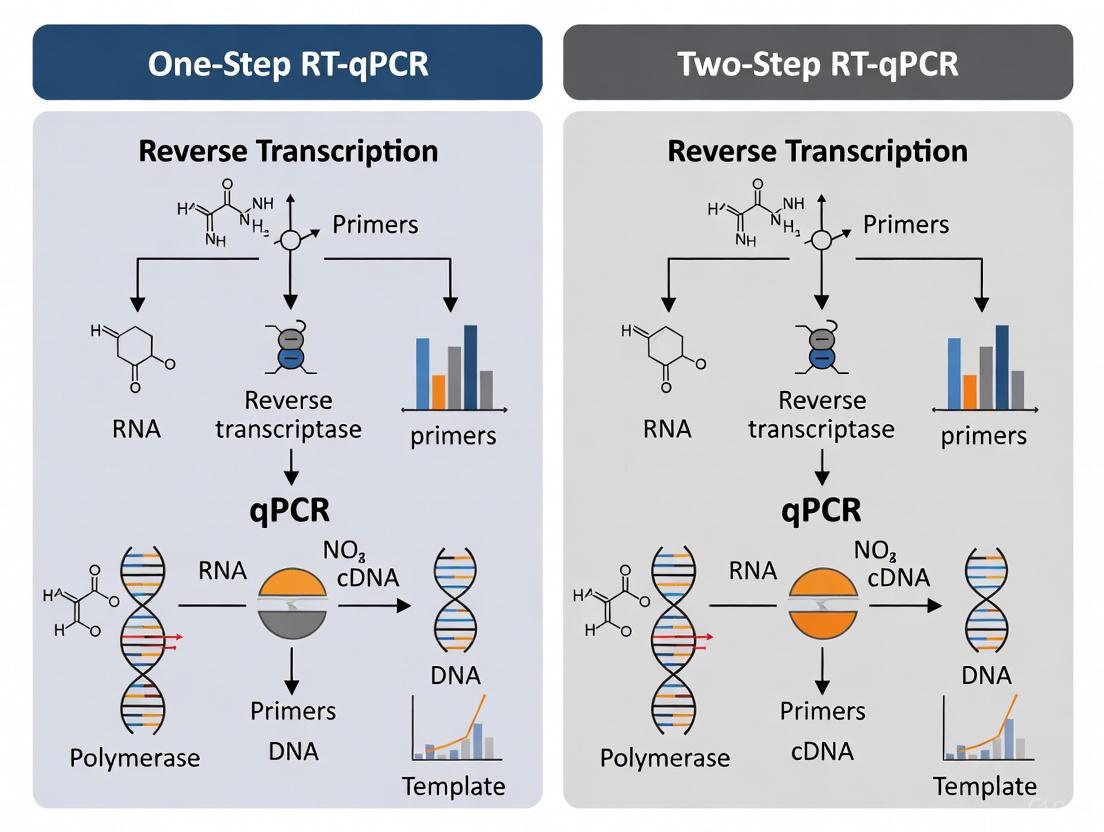
One-Step vs. Two-Step RT-qPCR: Choosing the Optimal Protocol for Cancer Biomarker Analysis
This article provides a comprehensive guide for researchers and drug development professionals on selecting between one-step and two-step RT-qPCR protocols for cancer biomarker applications.
Mastering Real-Time PCR Data Analysis: A Comprehensive Guide for Gene Expression Profiling in Biomedical Research
This comprehensive guide explores the foundational principles, methodologies, and best practices for real-time PCR data analysis in gene expression profiling.