Optimized RT-qPCR Protocol for Plasma miRNA Quantification: A Standardized Workflow for Reliable Biomarker Research
Circulating microRNAs in plasma have emerged as promising minimally invasive biomarkers for various diseases, from cancer to ageing-related disorders.
Optimized RT-qPCR Protocol for Plasma miRNA Quantification: A Standardized Workflow for Reliable Biomarker Research
Abstract
Circulating microRNAs in plasma have emerged as promising minimally invasive biomarkers for various diseases, from cancer to ageing-related disorders. However, the lack of standardization in RT-qPCR protocols has led to inconsistent results across studies, hindering clinical translation. This comprehensive guide addresses key challenges by providing an optimized, step-by-step workflow for reliable plasma miRNA quantification. It covers foundational principles, detailed methodological applications, essential troubleshooting strategies, and rigorous validation approaches. By integrating recent advancements in normalization methods, quality controls, and good laboratory practices, this protocol enables researchers and drug development professionals to generate reproducible, high-quality data essential for biomarker discovery and validation.
Circulating miRNAs as Biomarkers: Fundamental Principles and Analytical Challenges
The Biology and Stability of Circulating miRNAs in Plasma
MicroRNAs (miRNAs) are short (~22 nucleotides), non-coding RNA molecules that play a pivotal role in the post-transcriptional regulation of gene expression. Their presence in stable forms in biofluids like plasma and serum has generated significant interest for their potential as non-invasive biomarkers. Circulating miRNAs are remarkably stable under harsh conditions, including extreme pH shifts, enzymatic degradation, and multiple freeze-thaw cycles, which makes them exceptionally suitable for clinical diagnostics [1]. They are protected from endogenous RNase activity through their association with various carriers, including extracellular vesicles (e.g., exosomes), RNA-binding proteins (e.g., Argonaute 2), and lipoproteins [2] [3].
The development of blood-based methods for early disease detection is increasingly desirable across various medical fields. Circulating miRNA profiles hold significant promise as diagnostic biomarkers for a range of conditions, including cancer, autoimmune, liver, neurological, metabolic, and cardiovascular diseases [4] [5]. However, to realize their full potential in clinical applications, it is crucial to thoroughly characterize their stability under various blood collection, processing, and storage conditions and to establish robust, reproducible quantification protocols.
Stability of Plasma miRNAs
The analytical utility of any biomarker depends on its stability under common pre-analytical conditions. For circulating miRNAs, recent studies have systematically investigated their integrity in the face of variables encountered in routine clinical and research settings.
Stability Under Different Storage Conditions
Data demonstrate remarkable stability of miRNAs in both serum and plasma over time. Studies mimicking delays in processing encountered in routine clinical settings have shown that mean quantification cycle (Cq) values of specific miRNAs, such as miR-15b, miR-16, miR-21, miR-24, and miR-223, remain consistent between 0 and 24 hours when serum and plasma are stored on ice [4] [5]. Minimal changes are observed in mean Cq values over 24 hours even when serum is left at room temperature.
Small RNA-sequencing has detected approximately 650 different miRNA signals in plasma, with over 99% of the miRNA profile remaining unchanged even when blood collection tubes are left at room temperature for 6 hours prior to processing [4] [5]. This resilience demonstrates that miRNAs should withstand the variability in handling and processing that can occur with routine clinical lab draws.
Longitudinal Stability
The intraindividual longitudinal stability of miRNAs is a critical factor for their use in monitoring disease progression or treatment response. A comprehensive study collecting blood biweekly over a 3-month period from healthy adults identified 134 miRNAs with consistent amplification, 74 of which demonstrated high test-retest reliability and low percentage level drift, indicating they remain stable in an individual over time [2]. miRNAs with mean Cq values < 30.44 showed a low probability of unreliable amplification and lower within-participant standard deviation, making them more suitable for detecting subtle biological changes [2]. Of common confounding factors, hemolysis and tobacco use were found to have the greatest impact on miRNA levels and variance [2].
Table 1: Stability of Circulating miRNAs Under Various Conditions
| Condition | Findings | Key miRNAs Analyzed | Implication for Research |
|---|---|---|---|
| Short-term Storage (Room Temp & On Ice) | Mean Cq values remain consistent for 0-24h; >99% of miRNA profile unchanged after 6h at RT [4] [5] | miR-15b, miR-16, miR-21, miR-24, miR-223 | Withstands typical processing delays in clinical labs |
| Long-term Stability (3-month study) | 74 of 134 miRNAs showed high test-retest reliability and low level drift in individuals [2] | 74 stable miRNAs including miR-16-5p | Suitable for longitudinal disease monitoring |
| Pre-analytical Confounders | Hemolysis and tobacco use have the greatest impact on miRNA levels and variance [2] | N/A | Critical to record and control for these factors |
| Post-irradiation Stability | miRNAs remain stable and responsive as biomarkers of radiation exposure [1] | miR-126a-5p, miR-133a-3p | Useful as biomarkers even under physiological stress |
Absolute Quantification of Plasma miRNAs via RT-qPCR
While relative quantification is common, absolute quantification of plasma miRNAs is preferable for a comprehensive assessment of gene expression levels, as it allows for direct comparison of results across different studies and laboratories [6].
Detailed RT-qPCR Protocol
The following protocol describes the absolute quantification of plasma miRNAs using probe-based quantitative real-time reverse transcription PCR (RT-qPCR) with or without pre-amplification [6].
Sample Preparation and RNA Extraction
- Blood Collection and Plasma Isolation: Collect blood into EDTA-containing tubes (citrate and heparin are not acceptable as they inhibit subsequent PCR). Process samples for plasma isolation within 2 hours of collection. Centrifuge samples at 10,000 × g at 4°C for 5 min. Transfer the supernatant and centrifuge again at 16,000 × g at 4°C for 5 min to remove cell debris and residual platelets. Aliquot 200 µL of plasma into fresh microtubes and store at -80°C until use [6].
- RNA Extraction: Thaw samples on ice. Add 5 volumes (1000 µL) of lysis reagent (containing phenol and guanidine isothiocyanate) to the plasma sample (200 µL) and mix vigorously. Add 5 µL of 5 nM synthetic Caenorhabditis elegans miRNA (e.g., cel-miR-238-3p) as an external control for normalization. Add 1 volume (200 µL) of chloroform, mix vigorously, and centrifuge at 12,000 × g at 4°C for 15 min. Transfer the aqueous phase carefully to a new tube. Add 1.5 volumes of ethanol, mix, and transfer to a purification column. Wash and elute RNA in 50 µL of nuclease-free water [6].
cDNA Synthesis and Pre-amplification
- Standard Curve Preparation: Prepare a dilution series of synthetic RNA oligonucleotides identical to the target miRNAs. A suggested range for the standard curve is 1 x 10⁷ to 1 x 10² copies/µL for samples not requiring pre-amplification, or 1 x 10⁵ to 1 x 10⁰ copies/µL for pre-amplified samples [6].
- cDNA Synthesis: Prepare a multiplex RT primer pool by mixing equal volumes of 20x RT primers for target miRNAs. The external control (cel-miR-238) must be included in each reaction. Use up to 4 target miRNAs per pool. Perform reverse transcription according to kit specifications [6].
- Pre-amplification: For low-abundance miRNAs (Cq values above 35), perform pre-amplification prior to the qPCR step. This is crucial for enhancing detection of poorly expressed miRNAs [6].
Quantitative PCR and Data Analysis
- qPCR Setup: Perform probe-based qPCR using miRNA-specific forward/reverse PCR primers and probes.
- Absolute Quantification: Use the standard curve to determine the absolute copy number of each target miRNA in the original plasma sample. Normalize data using the Cq values of the spiked external control (cel-miR-238) to account for technical variations in RNA extraction and reverse transcription efficiency [6].
Critical Protocol Considerations
- Platelet Depletion: Quantification of miRNAs can be significantly affected by platelet contamination. The double-centrifugation protocol is essential to remove cell debris and residual platelets [6].
- Pre-amplification for Low-Abundance miRNAs: In a representative analysis of 8 miRNAs, miR-122, miR-133a, and miR-192 were detectable without pre-amplification, whereas miR-1, miR-206, and miR-499a required pre-amplification due to their low expression levels. MiR-208a and miR-208b were not detectable even after pre-amplification [6].
- Assay Performance: This method typically yields a technical variation of less than 3-fold and a lower limit of quantification (LLOQ) of 10² copies/µL for most examined miRNAs [6].
Advanced Technical Considerations
Normalization Strategies
A significant challenge in miRNA quantification is normalization. Using a fixed small RNA concentration input for reverse transcription, rather than a fixed RNA volume, provides superior quantification. Studies using droplet digital PCR (ddPCR) have shown that normalization based on small RNA concentration measurements (e.g., via Qubit miRNA assay) minimizes variations in eluted RNA concentrations that occur during extraction [7].
Addressing Hemolysis
Hemolysis in plasma significantly impairs the accurate detection of circulating cell-free miRNAs and is one of the most impactful confounders on miRNA levels and variance [7] [2]. It is essential to measure and report the hemolysis coefficient of plasma samples (e.g., at 414 nm) and to consider this in data analysis and interpretation.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Materials and Kits for Plasma miRNA Analysis
| Item | Function / Application | Example Products / Comments |
|---|---|---|
| Blood Collection Tubes | Plasma isolation for miRNA analysis | EDTA-containing tubes (avoid citrate/heparin) [6]; Streck tubes [8] |
| RNA Extraction Kits | Isolation of total RNA, including small RNAs, from plasma/serum | miRNeasy Serum/Plasma Kit (QIAGEN) [1] [3] [8]; MagnaZol cfRNA Isolation Reagent (Bioo Scientific) [9] |
| Carrier RNA | Improves RNA yield from low-concentration samples by aiding precipitation | MS2 bacteriophage carrier RNA (Roche) [7] |
| Synthetic Spike-in miRNAs | External control for normalization of technical variation from extraction to PCR | cel-miR-39-3p [2] [8], cel-miR-238-3p [6] |
| Small RNA Quantitation Assay | Accurate measurement of low-concentration small RNAs for input normalization | Qubit miRNA Assay (more specific than NanoDrop for small RNAs) [7] |
| Reverse Transcription & Preamplification Kits | cDNA synthesis and target enrichment for low-abundance miRNAs | TaqMan MicroRNA Reverse Transcription Kit, TaqMan PreAmp Master Mix (Applied Biosystems) [8] |
| Library Prep Kits (for NGS) | Preparation of small RNA libraries for next-generation sequencing | QIAseq miRNA Library Kit (QIAGEN), NEXTflex Small RNA-Seq Kit (Bioo Scientific), CleanTag Small RNA Library Prep Kit (TriLink) [9] |
| Absolute Quantification Technology | Digital PCR for direct copy number quantification without reference genes | Droplet Digital PCR (ddPCR) [7] |
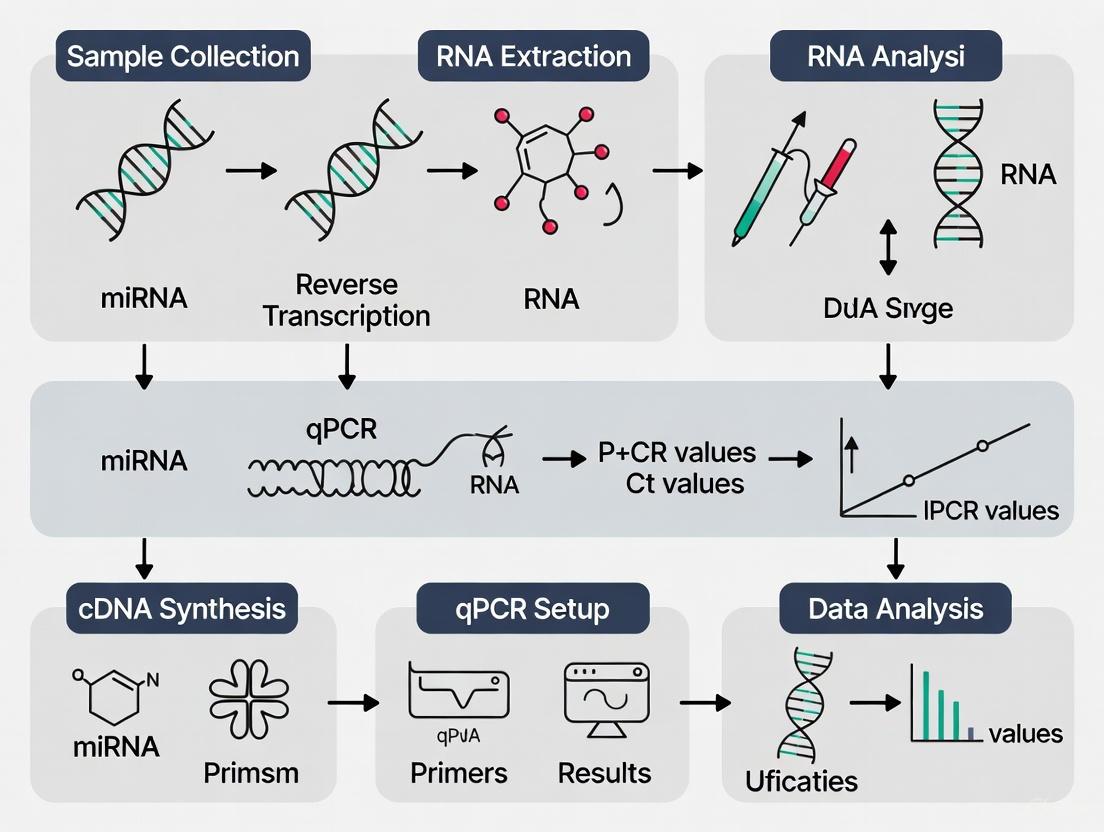
Experimental Workflow and Pathway Diagrams
Plasma miRNA Analysis Workflow
Diagram Title: Plasma miRNA RT-qPCR Workflow
Key Factors Influencing Plasma miRNA Data Quality
Diagram Title: Factors Affecting Plasma miRNA Data Quality
Circulating miRNAs in plasma represent a promising class of biomarkers due to their remarkable stability, association with physiological and pathological states, and accessibility through non-invasive blood draws. The RT-qPCR protocol outlined here, emphasizing absolute quantification with standard curves and careful normalization using synthetic spike-in controls, provides a robust framework for generating reliable and comparable data across laboratories. Attention to pre-analytical variables—especially blood collection methods, processing timelines, and hemolysis—is critical for success. As research in this field advances, the integration of these careful methodologies will be essential for translating the potential of circulating miRNAs into clinically valuable diagnostic and monitoring tools.
MicroRNAs (miRNAs) are small, non-coding RNA molecules that regulate gene expression post-transcriptionally and have emerged as promising biomarkers for ageing and age-related diseases [10] [11]. The ageing process is characterized by the accumulation of molecular and cellular damage, leading to functional decline and increased risk of chronic diseases [10]. Circulating cell-free miRNAs, released from cells into biological fluids, are remarkably stable in extracellular environments and are considered to reflect active physiological and pathological processes in the body, making them ideal candidates for non-invasive personalized molecular diagnosis [12]. The development of miRNA-based biomarkers is particularly valuable for age-related conditions like Alzheimer's disease (AD) and cancer, where early detection through minimally invasive methods could significantly improve patient outcomes [13] [14].
Cancer and ageing share numerous cellular pathways, including genomic instability, cellular senescence, and chronic inflammation [14]. However, while ageing typically leads to functional decline, cancer is characterized by uncontrolled cellular proliferation [14]. miRNAs serve as a unifying mechanism underlying the biology of both ageing and age-related conditions, interacting with various hallmarks of ageing such as DNA damage, cellular senescence, and mitochondrial dysfunction [10]. Understanding miRNA profiles in ageing and cancer opens pathways for therapeutic interventions and more effective prevention, detection, and treatment strategies [14].
Ageing-Associated miRNA Signatures and Their Clinical Utility
Population-Based miRNA Signatures in Aging
Recent large-scale population studies have identified distinct plasma miRNA signatures associated with ageing and health outcomes. A comprehensive study of 2,684 participants from the population-based Rotterdam Study cohort quantified 2,083 extracellular miRNAs and identified specific signatures linked to chronological age, frailty, and mortality [12]. The research led to the development of four plasma miRNA-based ageing biomarkers with significant clinical potential:
- mirAge: Comprising 108 miRNAs, trained on chronological age
- mirPA: Incorporating 153 miRNAs, trained on PhenoAge (a composite score of age and nine multi-system blood biomarkers)
- mirFI: Consisting of 81 miRNAs, trained on the frailty index
- mirMort: Including 50 miRNAs, trained on mortality risk
Elevated scores on these miRNA-based ageing biomarkers demonstrated robust associations with unfavorable health outcomes, including lower subjective physical functioning, poorer self-reported health, and increased mortality and frailty risk [12]. Notably, the effect estimates were larger for mirPA, mirFI, and mirMort compared to mirAge, suggesting that miRNAs trained on health status and mortality may be more informative than those trained solely on chronological age [12].
Dysregulation of miRNA Profiles in Disease States
Research comparing 1,334 healthy controls with 3,059 patients across various diseases (including Parkinson's disease, heart diseases, and lung cancer) revealed that the association between age and miRNA expression is partially lost in diseases [15]. Healthy controls showed approximately twice the absolute Spearman correlation for age-related miRNAs compared to the pooled disease cohort, suggesting that the presence of age-related diseases disrupts healthy ageing miRNA profiles [15]. This finding has significant implications for developing disease-specific biomarker panels.
Table 1: Key Ageing-Associated miRNA Clusters and Their Characteristics
| Cluster | Number of miRNAs | Correlation with Age | Representative Pathways Involved |
|---|---|---|---|
| Strongly Decreasing | 174 | SC < -0.2 | Negative correlation with age [15] |
| Moderately Decreasing | 382 | -0.2 < SC < -0.1 | Brain function, neurodegeneration [15] |
| Strongly Increasing | 174 | SC > 0.2 | Alzheimer's disease, synaptic transmission [15] |
| Moderately Increasing | 368 | 0.1 < SC < 0.2 | APP catabolic processes [15] |
miRNA Quantification Protocol for Plasma Samples
Sample Collection and Pre-analytical Processing
Standardized blood collection and processing is critical for reproducible miRNA quantification. The following protocol outlines best practices based on current research:
- Blood Collection: Collect fasting (8-14 hours) blood samples in EDTA Vacutainer tubes [12]. Process within 60 minutes using a standardized protocol [12].
- Plasma Separation: Centrifuge blood at 1,300 × g for 10 minutes in a 4°C refrigerated centrifuge for removal of formed elements [16]. Perform a second centrifugation at 3,000 × g for 10 minutes at 4°C to remove any residual platelets [16].
- Storage: Aliquot samples to minimize freeze-thaw cycles and store at -80°C [16] [17]. Extracted miRNAs remain stable for up to one year when stored at -70°C [17].
miRNA Isolation and Quality Control
- Isolation Method: Silica column-based RNA extraction methods are more effective and reliable compared to TRIzol LS [17]. Use commercial kits (e.g., miRNeasy mini kit) following manufacturer's recommendations with a starting volume of 100μL plasma or serum [16].
- Haemolysis Assessment: Assess sample quality using absorbance-based haemolysis detection or the RT-qPCR evaluation of miR-23a-3p and miR-451a [13]. Plasma samples with ΔCq (Cq of miR-23a-3p–Cq of miR-451a) <7 are considered clear of contamination [13].
- Spike-in Controls: Add synthetic cel-miR-39-3p (miRNeasy serum/plasma spiked-in control) during extraction to normalize miRNA isolation efficiency [16] [18]. Use 3.5μL at a concentration of 1.6×10^8 copies/μL [16].
Diagram 1: miRNA Quantification Workflow from Plasma
Reverse Transcription and Quantitative PCR
- Reverse Transcription: Use stem-loop RT primers specifically designed for miRNA quantification [16] [18]. Prepare a cDNA master mix containing dNTP Mix, RNase Inhibitor, RT Buffer, Multiscribe Reverse Transcriptase, and miRNA-specific RT Primers [18].
- Thermocycler Conditions: 5 minutes at 4°C, 30 minutes at 16°C, 30 minutes at 42°C, 5 minutes at 85°C, and hold at 4°C indefinitely with lid temperature at 105°C [18].
- qPCR Analysis: Perform using SYBR Green or TaqMan chemistry with appropriate controls [19]. Standard thermocycling conditions: initial denaturation at 95°C for 10 minutes, followed by 40 cycles at 94°C for 15 seconds and 60°C for 1 minute [19].
Normalization Strategies
Proper normalization is critical for accurate miRNA quantification. The following approaches are recommended:
- Exogenous Controls: Use spiked-in cel-miR-39 to normalize for differences in RNA isolation and reverse transcription efficiency [18] [17].
- Endogenous Normalizers: Implement a panel of stable normalizers verified in the specific experimental context. Recent research has identified 7 stable normalizers for ageing populations: let-7d-5p, let-7g-5p, let-7i-5p, miR-103a-3p, miR-107, miR-191-5p, and miR-423-5p [13].
- Novel Normalization Method: Utilize computational approaches like BestmiRNorm, developed using Python programming language, which enables assessment of up to 11 potential normalizers and allows researchers to weight evaluation according to their specific needs [13].
Table 2: Research Reagent Solutions for miRNA Quantification
| Reagent/Category | Specific Examples | Function | Protocol Notes |
|---|---|---|---|
| miRNA Isolation Kits | miRNeasy mini kit (Qiagen) | Silica column-based miRNA extraction | Use 100μL plasma/serum input volume [16] |
| Spike-in Controls | cel-miR-39-3p (Qiagen) | Normalization of isolation efficiency | Add 3.5μL at 1.6×10^8 copies/μL [16] |
| Reverse Transcription Kits | TaqMan miRNA RT Kit (Applied Biosystems) | cDNA synthesis from miRNA | Use stem-loop primers [18] |
| qPCR Reagents | SYBR Premix Ex Taq (Takara), TaqMan probes | miRNA quantification | VIC-MGB labeled probes for ddPCR [18] [19] |
| Reference miRNAs | let-7d-5p, let-7g-5p, miR-191-5p, etc. | Endogenous normalizers | 7 stable normalizers identified for ageing studies [13] |
Analytical Considerations and Technical Challenges
Pre-analytical Variables
Multiple factors affect circulating miRNA quantification and require careful standardization:
- Sample Type Comparison: Serum provides higher yields of certain miRNAs compared to plasma, with significant differences observed for miR-20a (p<0.0001) and miR-16-5p (p<0.0002) [16].
- Sample Storage: Endogenous circulating miRNA levels are unstable when plasma is stored at 4°C, requiring storage at -70°C for long-term stability [17].
- Haemolysis Detection: Absorbance-based methods provide reliable haemolysis assessment, which is crucial as haemolysed samples can significantly alter miRNA profiles [13].
Platform and Analytical Consistency
- Platform Effects: Significant variability in RT-qPCR results can be introduced by different machines and analysis software [13]. Studies demonstrate that the same sample run in parallel on different machines (StepOnePlus and 7900HT) and analyzed with different software packages (Sequence Detection System and ExpressionSuite Software) can yield significantly different results [13].
- Recommendation: Perform all steps of RT-qPCR analysis, including normalization, using the same machine and software throughout the entire study [13].
Diagram 2: Factors Influencing miRNA Quantification Accuracy
Clinical Applications in Ageing and Cancer
miRNA Biomarkers for Age-Related Diseases
Circulating miRNAs show particular promise as biomarkers for neurodegenerative diseases, cardiovascular conditions, and cancer:
- Alzheimer's Disease: miRNA biomarkers could enable early detection during protracted latent periods when diagnosis is challenging [13]. Blood-based miRNA diagnostics offer a less invasive alternative to current CSF-based biomarkers (amyloid Aβ, tau, and phosphorylated tau) and brain imaging methods [13].
- Cancer Detection: miRNA profiles demonstrate exceptional sensitivity and specificity for various cancers. For example, patients with thoracic aortic aneurysms have a unique plasma miRNA profile with exceptional diagnostic potential [18].
- Multi-disease Panels: Disease biomarker sets differ between young and old patients, necessitating age-specific validation of diagnostic panels [15].
Integration with Ageing Biomarkers
The relationship between miRNA signatures and established ageing biomarkers provides insights into biological ageing processes:
- PhenoAge Association: 227 miRNAs show robust associations with PhenoAge, a composite score of chronological age and nine multi-system blood biomarkers [12].
- Frailty Index: 61 miRNAs associate with the frailty index, indicating their role in age-related decline [12].
- Mortality Prediction: 16 miRNAs show association with 10-year mortality independent of chronological age [12].
Circulating miRNAs represent promising biomarkers for ageing and age-related diseases, offering insights into biological ageing processes and disease mechanisms. The development of standardized protocols for miRNA quantification—incorporating careful attention to pre-analytical variables, appropriate normalization strategies, and consistent analytical platforms—is essential for advancing their clinical application.
Future research directions should focus on validating miRNA biomarkers in diverse populations, establishing standardized protocols across laboratories, and integrating miRNA biomarkers with other omics technologies for a comprehensive understanding of ageing biology. The continued refinement of miRNA-based ageing biomarkers holds significant potential for early disease detection, risk stratification, and monitoring interventions aimed at promoting healthy ageing.
The analysis of circulating microRNAs (miRNAs) in plasma holds significant promise as a non-invasive approach for biomarker discovery in various human diseases [20]. MiRNAs are short (∼22 nucleotides), non-coding regulatory RNAs that are remarkably stable in extracellular environments, packaged within exosomes or complexed with proteins [20] [3]. Their stability, specificity to cell type and disease state, and detectability in biofluids make them excellent candidates for diagnostic and prognostic biomarkers across cancer, cardiovascular, metabolic, and neurological disorders [20] [12]. However, the quantification of plasma miRNAs is technically challenging and fraught with numerous sources of variability that can significantly impact data reproducibility and interpretation [18]. This application note details the major sources of variability in plasma miRNA analysis and provides standardized protocols to enhance data reliability within the broader context of thesis research on RT-qPCR protocol development for miRNA quantification.
The variability in plasma miRNA analysis can be categorized into three main phases: pre-analytical, analytical, and post-analytical. The schematic diagram below illustrates the complete workflow and key decision points.
Pre-Analytical Variability
Pre-analytical factors constitute the most significant source of variability in plasma miRNA analysis, introducing inconsistencies before measurement even begins.
Table 1: Pre-Analytical Variables and Their Impact on miRNA Quantification
| Variable | Specific Factors | Impact on miRNA Profiles | Recommended Protocol |
|---|---|---|---|
| Blood Collection Tube | EDTA plasma vs. Serum tubes | Serum provides higher miRNA yields than plasma [16]; different stabilization chemistries | Standardize tube type across study; K₂EDTA for plasma, clotting tubes for serum [20] |
| Processing Delay | Time between blood draw and processing (0-24 h) | Minimal changes in mean Cq values over 24h at room temperature [20]; >99% miRNA profile unchanged at 6h [20] | Process within 1h; if delayed, store on ice or at 4°C for up to 24h [20] |
| Centrifugation Protocol | Speed, duration, temperature | Incomplete cell removal contaminates with cellular miRNAs; platelet-rich plasma alters profiles | Two-step centrifugation: 1200×g for 10min, then 1500×g for 5min at room temperature [20] |
| Storage Conditions | Temperature, freeze-thaw cycles | Degradation with repeated freeze-thaw; stable at -80°C long-term | Aliquot to avoid repeated freeze-thaw; store at -80°C [18] [3] |
Analytical Variability
Analytical variability arises from technical differences in miRNA isolation, quantification, and amplification methodologies.
RNA Isolation and Quality Control
The efficiency of miRNA isolation varies substantially between methods and sample volumes. Studies indicate that using 100μL of plasma or serum provides optimal recovery, with smaller or larger volumes yielding undetectable or suboptimal miRNA levels [16]. The inclusion of exogenous spike-in controls like cel-miR-39-3p is crucial for normalizing extraction efficiency [18] [16]. RNA concentrations typically range from 0.25-1.0 ng/μL after extraction from 100μL plasma [16].
Quantification Methodologies
Both RT-qPCR and droplet digital PCR (ddPCR) are widely used for miRNA quantification, each with distinct advantages and limitations.
Table 2: Comparison of miRNA Quantification Platforms
| Platform | Multiplexing Capacity | Sensitivity | Quantitative Output | Best Application |
|---|---|---|---|---|
| RT-qPCR | Low to moderate (typically 4-6 targets) | High (detects single copies) | Relative quantification (Cq values) | Targeted analysis of known miRNAs; high-throughput screening [3] |
| ddPCR | Moderate | Very high; absolute quantification without standard curves | Absolute copy numbers | Validation studies; detection of low-abundance miRNAs [18] |
| Small RNA-Seq | High (∼650 different miRNA signals detected) [20] | Moderate | Counts per million (CPM) | Discovery phase; novel miRNA identification [21] [12] |
| Color Cycle Multiplex Amplification (CCMA) | Very high (theoretically 136 targets with 4 colors) [22] | High | Fluorescence permutation patterns | Syndromic testing; pathogen identification [22] |
Post-Analytical Variability
Data normalization represents a critical post-analytical challenge in plasma miRNA analysis. The lack of universally accepted reference miRNAs necessitates careful validation of endogenous controls or implementation of exogenous normalization strategies.
Common normalization approaches include:
- Exogenous spike-ins: Synthetic non-human miRNAs (e.g., cel-miR-39) added at the beginning of RNA isolation [18] [16]
- Endogenous controls: Consistently expressed human miRNAs (e.g., miR-16-5p, miR-24) identified through stability testing [20] [3]
- Global mean normalization: Averaging expression across all detected miRNAs, preferred in sequencing studies [12]
Standardized Protocol for Plasma miRNA Quantification
Blood Collection and Plasma Processing
- Blood Collection: Draw blood into K₂EDTA tubes (for plasma) or clotting tubes (for serum). In a study of healthy volunteers, plasma and serum were collected in 10mL tubes [20].
- Processing Timeline: Process samples within 1 hour of collection. If delays are anticipated, store samples on ice or at 4°C for up to 24 hours, as miRNAs demonstrate remarkable stability under these conditions [20].
- Centrifugation Protocol:
- Centrifuge at 1200×g for 10 minutes at room temperature
- Carefully transfer the supernatant to a new tube
- Centrifuge at 1500×g for an additional 5 minutes at room temperature
- Aliquot plasma/serum into 0.5mL portions [20]
- Storage: Store aliquots at -80°C to preserve miRNA integrity
miRNA Isolation with Quality Control
- Sample Volume: Use 100μL of plasma or serum for RNA isolation, as this volume provides optimal recovery [16].
- Spike-in Control: Add 3.5μL of synthetic cel-miR-39-3p (1.6×10⁸ copies/μL) to each sample prior to extraction [16].
- Extraction Method: Use the miRNeasy Serum/Plasma Advanced Kit (Qiagen) or similar phenol-chloroform based isolation systems [3].
- Elution: Elute RNA in 30-50μL of RNase-free water [16].
- Quality Assessment: Measure RNA concentration using fluorometric methods (e.g., Qubit microRNA assay); expected yields range from 0.25-1.0 ng/μL [16].
Reverse Transcription and qPCR
- Reverse Transcription: Use the TaqMan Advanced miRNA cDNA Synthesis Kit following manufacturer's protocols, which includes poly(A) tailing and adapter ligation [3].
- qPCR Setup:
- Use TaqMan Fast Advanced Master Mix
- Set up reactions in triplicate
- Include no-template controls
- Use the following cycling conditions: 95°C for 20 seconds, followed by 40 cycles of 95°C for 1 second and 60°C for 20 seconds [3]
- Data Analysis:
- Determine Cq values using the instrument's software
- Normalize data using the ΔΔCq method with reference to spike-in controls (cel-miR-39) and/or validated endogenous controls
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Plasma miRNA Analysis
| Reagent | Function | Example Products | Critical Notes |
|---|---|---|---|
| RNA Isolation Kit | Small RNA enrichment from biofluids | miRNeasy Serum/Plasma Kit (Qiagen) | Includes spike-in control; optimized for volumes ≤100μL [16] |
| Exogenous Spike-in Control | Normalization of extraction efficiency | cel-miR-39-3p (Qiagen) | Add at beginning of isolation; non-human sequence [18] [16] |
| Reverse Transcription Kit | cDNA synthesis from mature miRNAs | TaqMan Advanced miRNA cDNA Synthesis Kit | Includes poly(A) tailing and adapter ligation [3] |
| qPCR Master Mix | Fluorescence-based detection | TaqMan Fast Advanced Master Mix | Probe-based chemistry for specific detection [3] |
| Reference miRNAs | Endogenous normalization | miR-16-5p, miR-24, miR-223 | Must be validated for stability in each study context [20] |
| Droplet Digital PCR | Absolute quantification without standard curves | Bio-Rad QX200 System | Provides copy number quantification; superior precision [18] |
The analysis of plasma miRNAs presents unique challenges with multiple sources of variability spanning pre-analytical, analytical, and post-analytical phases. The remarkable stability of miRNAs in plasma—maintaining integrity for up to 24 hours under various storage conditions—makes them promising biomarker candidates, but this advantage can only be leveraged through stringent protocol standardization [20]. Key considerations include uniform sample processing, implementation of exogenous controls, and appropriate normalization strategies. The protocols and guidelines presented here provide a framework for reliable plasma miRNA quantification that will enhance data reproducibility and facilitate the translation of circulating miRNA biomarkers into clinical applications.
The reliability of circulating microRNA (miRNA) analysis in plasma research is highly dependent on the pre-analytical phase. Variations in sample collection, processing, and storage introduce significant confounding variability that can compromise data integrity and reproducibility for RT-qPCR protocols [23] [24]. This application note details standardized procedures to control these critical pre-analytical factors, providing a robust foundation for miRNA quantification within a broader thesis on RT-qPCR methodology.
Critical Pre-analytical Variables & Experimental Data
Sample Collection: Plasma vs. Serum
The choice of sample matrix fundamentally influences miRNA concentration. Studies consistently demonstrate differential miRNA yields between plasma and serum, influenced by the coagulation process.
Table 1: Comparison of miRNA Levels in Paired Plasma and Serum Samples from Healthy Cats (n=10) [16]
| microRNA | Mean Ct in Plasma (Mean ± SE) | Mean Ct in Serum (Mean ± SE) | P-value |
|---|---|---|---|
| miR-20a | 26.4 ± 0.4 | 28.5 ± 0.5 | < 0.0001 |
| miR-16-5p | 21.6 ± 0.4 | 24.3 ± 0.6 | < 0.0002 |
| miR-192 | 29.2 ± 0.4 | 29.9 ± 0.4 | Not Significant |
| miR-365 | 28.8 ± 0.5 | 30.5 ± 0.6 | Not Significant |
| miR-15b-5p | 30.2 ± 0.3 | 30.9 ± 0.5 | Not Significant |
Experimental Protocol: Plasma vs. Serum Comparison [16]
- Blood Collection: Venous blood was drawn from 10 healthy domestic cats.
- Sample Separation: Blood was collected into:
- K3EDTA-containing tubes for plasma.
- Non-additive tubes for serum.
- Processing:
- Serum: Tubes were centrifuged at 1,814 × g for 5 minutes. Serum was carefully aspirated to avoid cellular debris.
- Plasma: K3EDTA tubes were centrifuged at 1,300 × g for 10 minutes at 4°C. A second centrifugation at 3,000 × g for 10 minutes at 4°C was performed to remove residual platelets.
- miRNA Quantification: RNA was extracted from 100 µL aliquots using the miRNeasy mini kit (Qiagen) with cel-miR-39-3p spike-in control. Five target miRNAs were quantified via RT-rtPCR in triplicate.
Blood Processing and Centrifugation
Centrifugation protocols are critical for defining the "cell-free" fraction and minimizing contamination from platelets and cellular miRNAs.
Table 2: Impact of Centrifugation Protocols on Plasma miRNA Analysis [23] [24]
| Factor | Recommended Protocol | Impact on miRNA Profile |
|---|---|---|
| Primary Centrifugation | 820 - 3,500 × g for 1-20 min at +4°C or RT [23].2,000 × g for 10 min (standard plasma) [24]. | Removes leukocytes and the majority of platelets. |
| Secondary Centrifugation | 10,000 - 16,000 × g for 15 min [23].3,000 × g for 15 min (platelet-poor plasma) [24]. | Significantly reduces platelet-associated miRNAs (e.g., miR-24, miR-191, miR-197) [23]. |
| Protocol Comparison | Dual centrifugation vs. prolonged single (3,000 × g, 30 min) [24]. | Similar miRNA levels were found in platelet-poor plasma from both protocols. Poor correlation was observed between standard and platelet-poor plasma. |
Sample Storage and miRNA Stability
Circulating miRNAs demonstrate remarkable stability under various conditions, a key advantage for their use as biomarkers.
Table 3: miRNA Stability Under Different Storage Conditions [20] [23]
| Condition | Findings | Experimental Summary |
|---|---|---|
| Short-Term Stability (0-24h) | miRNAs (e.g., miR-15b, miR-16, miR-21, miR-24, miR-223) in serum and plasma showed consistent mean Cq values when stored on ice or at room temperature [20]. | Protocol: Plasma/serum from healthy volunteers stored at 4°C or 25°C for 0-24h. miRNA profiles assessed by RT-qPCR and small RNA-seq. Small-RNA sequencing detected ~650 miRNAs, with >99% unchanged after 6h at RT [20]. |
| Long-Term Storage | Stable in serum/plasma for at least 1 year at -20°C or -80°C [23]. Samples stored at -20°C provide similar results to -80°C [23]. | Grasedieck et al. showed frozen samples remain stable for several years with comparable results from -20°C and -80°C storage [23]. |
| Freeze-Thaw Cycles | Should be avoided [23]. Aliquot samples to minimize repeated thawing [16]. | Temperature changes during freeze-thaw cycles reduce available miRNA molecules [16]. |
Quality Control: Hemolysis Assessment
Hemolysis is a major source of pre-analytical variation, as red blood cells contain high concentrations of specific miRNAs.
Experimental Protocol: Hemolysis Evaluation [23]
- Spectrophotometric Method: Measure absorbance of plasma/serum at 414 nm. An absorbance value > 0.2 indicates a hemolyzed sample that should be excluded.
- miRNA Ratio Method: Calculate the miR-451/miR-23 ratio. A high value indicates hemolysis, as miR-451 is abundant in red blood cells.
- Hemolytic Index (HI): Use standard biochemical platforms to measure HI. Higher values indicate greater cell-free hemoglobin concentration.
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Materials for Plasma miRNA Analysis
| Item | Function | Example Products & Notes |
|---|---|---|
| K2/K3EDTA Blood Tubes | Anticoagulant for plasma preparation. Preferred over heparin (inhibits PCR) and citrate (can cause hemolysis) [23]. | Becton-Dickinson [24]. |
| Specialized ccfDNA Tubes | Preserve cell-free RNA profile by preventing cell lysis in whole blood for up to 7 days at RT [23]. | PAXgene Blood ccfDNA Tubes (Qiagen), Cell-Free DNA Collection Tubes (Roche) [23]. |
| miRNA Extraction Kits | Optimized for low-concentration, high-purity isolation of small RNAs from plasma/serum. | miRNeasy Serum/Plasma Kit (Qiagen) [16] [20], mirVana (Thermo Fisher) [23]. |
| Spike-in Control (cel-miR-39) | Exogenous normalization control for quality control of extraction and reverse transcription efficiency [25] [26]. | miRNeasy serum/plasma spiked-in control (Qiagen) [16]. |
| Endogenous Normalizers | Stable endogenous miRNAs used for data normalization to correct for biological variance. | Requires validation for specific study context. miR-16-5p is common but not universal [25] [2]. hsa-miR-205-3p was stable in COVID-19 vs. controls [25]. |
Visual Workflow for Pre-analytical Phase
The following diagram summarizes the standardized workflow for plasma processing, from blood draw to analysis, incorporating critical decision points and quality control checks.
Detailed Experimental Protocols
Objective: To obtain plasma with minimal platelet contamination for miRNA analysis. Reagents & Equipment:
- K2EDTA blood collection tubes.
- Refrigerated centrifuge.
- Transfer pipettes.
- Cryo-tubes for storage.
Procedure:
- Blood Collection: Collect venous whole blood using a 21-gauge needle into K2EDTA tubes after discarding the first 3 mL of blood. Minimize venous stasis.
- Primary Centrifugation: Centrifuge the whole blood tubes at 3,000 × g for 15 minutes at 18°C (acceleration 5, brake 6).
- Initial Plasma Transfer: Carefully transfer the plasma phase to a new centrifuge tube, leaving approximately 1 mL of plasma on top of the buffy coat to avoid cellular contamination.
- Secondary Centrifugation: Recentrifuge the transferred plasma at 3,000 × g for 15 minutes at 18°C.
- Final Plasma Transfer: Carefully transfer the resulting platelet-poor plasma (PPP) into cryo-tubes, again leaving about 1 mL in the bottom of the tube.
- Storage: Store PPP aliquots at -80°C within 2 hours of blood collection.
Objective: To verify the stability of circulating miRNA profiles in plasma under different processing and storage delays. Reagents & Equipment:
- K2EDTA tubes (plasma) or clotting tubes (serum).
- miRNeasy Serum/Plasma Kit (Qiagen).
- RT-qPCR reagents and instrumentation.
- Thermocycler.
Procedure:
- Sample Collection and Processing: Collect whole blood from healthy volunteers. For plasma, centrifuge at 1,200 × g for 10 min at room temperature (RT). For serum, allow to clot for 30 min at RT before centrifugation.
- Aliquot and Storage Conditions: Aliquot plasma/serum samples (0.5 mL) into microcentrifuge tubes.
- For time-course experiments, leave aliquots for 0, 6, and 24 hours on ice or at room temperature before freezing.
- For processing delay simulation, leave whole blood at RT for 0, 2, and 6 hours before plasma isolation.
- RNA Isolation and Analysis: Isolate miRNA from all samples using the miRNeasy kit. Assess miRNA profiles using RT-qPCR for specific targets (e.g., miR-15b, miR-16, miR-21, miR-24, miR-223) or small RNA-sequencing for untargeted profiling.
- Data Analysis: Compare mean Cq values and global miRNA profiles across time points and conditions to assess stability.
Understanding the RT-qPCR Workflow for Low-Abundance Targets
Reverse Transcription quantitative Polymerase Chain Reaction (RT-qPCR) is a powerful technique for quantifying RNA molecules, prized for its sensitivity, specificity, and quantitative capabilities [27]. The analysis of low-abundance targets, such as specific microRNAs (miRNAs) in plasma, presents a particular challenge. Their low concentration, combined with the complex background of human plasma, demands a rigorously optimized and validated workflow to generate reliable, reproducible data. This application note details a refined RT-qPCR protocol, framed within miRNA plasma research, to guide researchers and drug development professionals in the accurate quantification of these challenging targets.
The RT-qPCR Workflow: A Step-by-Step Guide
The journey from blood sample to quantifiable gene expression data involves a series of critical steps, each of which must be carefully controlled to preserve the integrity of low-abundance RNA targets.
Sample Collection and RNA Stabilization
The foundation of any successful RT-qPCR experiment is high-quality starting material. For circulating miRNA analysis, blood samples are typically collected in EDTA tubes (for plasma) or clotting tubes (for serum) [20]. Pre-analytical factors are crucial; delays in processing or improper storage can compromise RNA quality. Encouragingly, miRNAs demonstrate remarkable stability. Studies show that mean Cq values for specific miRNAs (e.g., miR-15b, miR-16, miR-21) remain consistent for up to 24 hours when plasma or serum is stored on ice or even at room temperature, making them robust analytes for clinical settings [20]. After collection, plasma or serum is isolated via centrifugation and aliquots should be stored at -80°C to preserve RNA until extraction [3].
RNA Extraction and Quality Control
RNA, including small miRNAs, is extracted from plasma or plasma-derived exosomes using specialized kits designed for low-concentration biofluids, such as the miRNeasy Serum/Plasma Advanced Kit [3]. The inclusion of a spike-in control, like cel-miR-39, during the extraction process is a critical step for normalizing technical variations related to extraction efficiency and allows for more accurate relative quantification [19]. RNA concentration and purity should be assessed using a spectrophotometer (e.g., Nanodrop), though for low-yield plasma samples, the concentration may be very low [3].
Reverse Transcription (RT)
The RT reaction converts RNA into more stable complementary DNA (cDNA). This process requires several key reagents: primers, reverse transcriptase, dNTPs, MgCl₂, and RNase inhibitors [27]. The choice of RT primer is a key decision point. For miRNA quantification, a common and effective approach is to use a universal RT primer following poly(A) tailing and adapter ligation of the miRNAs, which provides a uniform method to reverse transcribe all miRNAs [3]. For mRNA targets, gene-specific primers offer high sensitivity, while random primers or oligo(dT) primers are used for broader transcript coverage [27]. The reaction involves denaturing RNA secondary structures, primer annealing, cDNA synthesis, and enzyme inactivation [27].
Quantitative PCR (qPCR)
In the qPCR step, the cDNA is amplified and quantified in real-time. The reaction mixture contains DNA polymerase, sequence-specific primers, dNTPs, and a fluorescent reporter system [27]. For low-abundance targets, primer design is paramount. Key considerations include:
- Length: 18-25 nucleotides for standard primers [27]; 15-30 nucleotides for miRNA assays [28].
- GC content: 40-60% for stable binding [27] [28].
- Specificity: Design primers to span exon-exon junctions where applicable to avoid genomic DNA amplification [27]. Use tools like NCBI BLAST to ensure specificity.
- Amplicon length: Short amplicons (70-200 bp) are recommended for maximum PCR efficiency, which is especially important for low-abundance targets [27] [28].
Thermal cycling involves an initial denaturation (e.g., 95°C for 20 seconds), followed by 40-45 cycles of denaturation, primer annealing (typically 55-65°C), and extension [27] [3] [19]. Fluorescence is measured at each cycle, generating an amplification curve.
Data Analysis
Quantification is based on the Cycle Threshold (Ct) value, the cycle number at which the fluorescent signal crosses a defined threshold [27]. A low Ct value indicates a high starting quantity of the target. For relative quantification of miRNA in plasma, the comparative Cq (ΔΔCq) method is often used, where the target miRNA expression is normalized to a spike-in control (like cel-miR-39) and compared to a control group [19].
Figure 1: Core RT-qPCR workflow for plasma miRNA analysis. Critical sample preparation and stabilization steps are highlighted in red.
Critical Experimental Protocols
Protocol 1: Plasma miRNA Extraction and RT-qPCR
This protocol is adapted from a study profiling plasma miRNAs in patients with medication-related osteonecrosis of the jaw [3] and a bio-protocol for quantifying plasma miRNA levels [19].
Sample Preparation:
- Collect whole blood in K₂EDTA tubes (for plasma) and centrifuge at 1,200-1,500 × g for 10 minutes at room temperature.
- Carefully collect the top plasma layer and centrifuge again at 1,500 × g for 5 minutes to remove any residual cells.
- Aliquot plasma and store at -80°C.
miRNA Extraction:
- Extract miRNA from plasma using a specialized kit (e.g., miRNeasy Serum/Plasma Advanced Kit, Qiagen) according to the manufacturer's instructions.
- Spike-in Control: Add a known quantity of synthetic non-human miRNA (e.g., cel-miR-39) to the lysis buffer prior to RNA extraction to control for variations in extraction efficiency and downstream reactions [19].
Reverse Transcription (with Poly-A Tailing):
- Use a cDNA synthesis kit (e.g., TaqMan Advanced miRNA cDNA Synthesis Kit) [3].
- Perform poly(A) tailing and adapter ligation on the miRNA, followed by reverse transcription with universal RT primers.
Quantitative PCR:
- Reaction Setup: Use a probe-based or dye-based master mix. A typical 20 µl reaction contains 1X master mix, primers (400 nM recommended), probe if applicable (200 nM recommended), and cDNA template.
- Thermocycling Conditions:
Protocol 2: Analytical Validation for Low-Abundance Targets
To ensure confidence in your data, particularly for low-abundance targets, key analytical performance characteristics of the RT-qPCR assay must be validated [29] [30].
1. Determine Amplification Efficiency and Dynamic Range:
- Prepare a 7-point, 10-fold serial dilution series of a sample or synthetic target with known concentration.
- Run each dilution in triplicate on the qPCR platform.
- Plot the log of the starting template concentration against the Ct value.
- A slope between -3.1 and -3.6 (90-110% efficiency) and a coefficient of determination (R²) ≥ 0.99 are indicative of a highly efficient and linear assay [28] [30].
2. Assess Assay Specificity (Exclusivity):
- Perform in silico analysis using primer design software and databases (e.g., NCBI BLAST) to ensure primers do not bind to non-target sequences.
- Test the assay experimentally against a panel of non-target RNAs to check for cross-reactivity [30].
- For dye-based chemistries, perform melt curve analysis post-amplification to verify a single, specific amplification product [31].
3. Establish the Limit of Detection (LOD) and Quantification (LOQ):
- The LOD is the lowest concentration at which the target can be reliably detected. The LOQ is the lowest concentration that can be quantified with acceptable precision and accuracy [30].
- These are determined by repeatedly testing low concentrations of the target and identifying the concentration where detection is ≥95% probable (LOD) and where the % coefficient of variation is within an acceptable limit (e.g., < 35%) for LOQ.
Table 1: Key Performance Characteristics for a Validated RT-qPCR Assay
| Parameter | Target Value | Importance for Low-Abundance Targets |
|---|---|---|
| Amplification Efficiency | 90–110% [28] [30] | Ensures accurate quantification; inefficient assays underestimate low-concentration targets. |
| Linear Dynamic Range | 6–8 orders of magnitude [30] | Confirms the assay is quantitative across a wide range of concentrations, including low levels. |
| Linearity (R²) | ≥ 0.99 [28] [30] | Indicates a strong, linear relationship between input and Ct value. |
| Assay Specificity | Single peak in melt curve or specific probe detection [31] | Ensures the signal comes from the intended target and not from nonspecific amplification. |
| Precision (Repeatability) | CV < 5% for Cq values [29] | Confirms the assay produces consistent results when repeated. |
The Scientist's Toolkit: Essential Reagents and Materials
Table 2: Key Research Reagent Solutions for Plasma miRNA RT-qPCR
| Reagent / Kit | Function | Example Product / Note |
|---|---|---|
| RNA Extraction Kit | Isolates total RNA, including small RNAs, from biofluids. | miRNeasy Serum/Plasma Advanced Kit (Qiagen) [3]. |
| Spike-in Control | Synthetic RNA added to sample to normalize for technical variation. | cel-miR-39 [19]. Critical for plasma miRNA studies. |
| Reverse Transcriptase | Enzyme that synthesizes cDNA from an RNA template. | Components in TaqMan Advanced miRNA cDNA Synthesis Kit [3]. |
| qPCR Master Mix | Contains DNA polymerase, dNTPs, buffer, and fluorescent dye/probe. | SYBR Green or TaqMan Probe Master Mixes (e.g., from Applied Biosystems, NEB) [31] [28]. |
| Sequence-Specific Primers | Oligonucleotides that define the target region for amplification. | Designed with 40-60% GC content; Tm ~60°C [27] [28]. |
| Hydrolysis Probes | Fluorescently-labeled probes for specific target detection. | TaqMan Probes (5' nuclease assay); double-quenched probes recommended for better signal-to-noise [31] [28]. |
| Universal Passive Reference Dye | Normalizes for non-PCR-related fluorescence fluctuations. | Included in many commercial master mixes (e.g., ROX) [28]. |
Figure 2: Essential reagents and their roles in the RT-qPCR workflow, grouped by experimental phase.
The accurate quantification of low-abundance miRNA targets in plasma via RT-qPCR is an achievable goal when a meticulous, validated workflow is followed. Success hinges on careful attention to pre-analytical sample handling, the use of appropriate normalization controls (e.g., spike-ins), rigorous primer and assay design, and thorough analytical validation. By adhering to the detailed protocols and guidelines outlined in this document, researchers can generate robust, reproducible, and clinically meaningful data that advances our understanding of disease mechanisms and biomarkers.
Step-by-Step Optimized RT-qPCR Protocol for Plasma miRNA Analysis
Within the framework of developing an RT-qPCR protocol for microRNA (miRNA) quantification, the pre-analytical phase of blood collection and processing is a critical determinant of success. The choice between plasma and serum, along with the appropriate anticoagulant, directly impacts miRNA stability, yield, and the overall reliability of downstream molecular analyses. This document provides detailed application notes and protocols for blood sample collection, specifically optimized for miRNA quantification in plasma via RT-qPCR, to support robust and reproducible biomarker research and drug development.
Plasma vs. Serum: A Comparative Analysis for miRNA Research
The selection of the blood matrix is a fundamental first step. While both plasma and serum are acellular fractions, their methods of preparation lead to significant differences in their composition and suitability for miRNA analysis.
Table 1: Comparative Analysis of Plasma and Serum for miRNA Profiling
| Feature | Plasma | Serum |
|---|---|---|
| Definition | Liquid fraction of blood maintained in its natural state with anticoagulants. | Liquid fraction of blood obtained after clotting has occurred. |
| Clotting | Prevented by anticoagulant additives (e.g., K₂EDTA, citrate). | Occurs naturally, consuming platelets and various coagulation factors. |
| miRNA Yield | Generally higher and more consistent; avoids miRNA entrapment in the clot [20]. | Variable; can be lower due to retention of miRNAs in the clot and platelets. |
| Composition | Contains fibrinogen, other clotting factors, and circulating miRNAs. | Lacks fibrinogen; contains proteins and miRNAs released from platelets during clotting. |
| Handling | Requires immediate centrifugation post-collection to separate cells. | Requires a clotting period (typically 30 mins) prior to centrifugation [20]. |
| Key Advantage | Minimizes cellular miRNA contamination and offers a more representative profile of circulating miRNAs. | -- |
| Key Disadvantage | Potential for hemolysis if processed incorrectly; anticoagulant can interfere with some downstream assays. | Platelet activation during clotting can alter the miRNA profile, introducing variability [2]. |
Recent studies underscore the remarkable stability of miRNAs in both plasma and serum. Research demonstrates that specific miRNAs (e.g., miR-15b, miR-16, miR-21, miR-24, miR-223) remain stable with minimal changes in quantification cycle (Cq) values when samples are stored on ice or at room temperature for up to 24 hours [20]. Small RNA-sequencing data further confirms that over 99% of the miRNA profile remains unchanged in plasma even when blood collection tubes are left at room temperature for 6 hours before processing [20]. This stability is attributed to the protection of miRNAs by packaging within exosomes or complexing with proteins [20]. Furthermore, longitudinal studies have identified 74 miRNAs with high test-retest reliability and low drift in plasma from healthy adults over a 3-month period, reinforcing their suitability as stable biomarkers [2].
Anticoagulant Selection Guide
The choice of anticoagulant in plasma collection tubes is crucial, as it can affect viscosity, RNA extraction efficiency, and PCR chemistry.
Table 2: Common Anticoagulants in Blood Collection for miRNA Studies
| Anticoagulant | Tube Color (Top) | Mechanism of Action | Considerations for RT-qPCR |
|---|---|---|---|
| K₂EDTA | Lavender / Purple | Chelates calcium ions. | Preferred choice. Minimal interference with RT-qPCR. Avoids dilution of sample. Check for inhibition in downstream assays. |
| Citrate | Light Blue | Chelates calcium ions. | Causes sample dilution (~1:9 ratio), which may dilute miRNA targets. Can affect calcium-dependent enzymes in some assay systems. |
| Heparin | Green | Activates antithrombin III. | Not recommended. Heparin is a potent inhibitor of reverse transcriptase and Taq polymerase, leading to severe suppression or complete failure of PCR amplification. |
Detailed Experimental Protocols
Standardized Protocol for Plasma and Serum Separation
The following protocol is adapted from established methodologies to ensure miRNA integrity [20].
Materials:
- K₂EDTA tubes (for plasma) and clotting tubes (for serum)
- Centrifuge
- Micropipettes and sterile, nuclease-free tips
- Nuclease-free microcentrifuge tubes
- Personal protective equipment
Procedure:
- Blood Draw: Perform venipuncture and collect blood into the appropriate vacutainer tubes. Invert K₂EDTA tubes 8-10 times gently to mix the anticoagulant.
- Clotting (Serum only): For serum tubes, leave the blood sample at room temperature for 30 minutes to allow complete clot formation [20].
- Initial Centrifugation: Centrifuge both plasma and serum tubes at 1,200 × g for 10 minutes at room temperature.
- Liquid Transfer: Carefully transfer the upper liquid layer (plasma or serum) to a new nuclease-free tube using a micropipette, avoiding the buffy coat (white cell layer) and the clot (in serum) or cells (in plasma).
- Secondary Centrifugation: Centrifuge the transferred liquid at a higher speed of 1,500 × g for 5 minutes at room temperature to remove any remaining cells or debris [20].
- Aliquoting: Transfer the supernatant into fresh nuclease-free tubes in small, single-use aliquots to avoid repeated freeze-thaw cycles.
- Storage: Store aliquots at -80°C until RNA extraction.
Pre-analytical Stability: As validated, plasma and serum samples for miRNA analysis can be left on ice or at room temperature for 0–24 hours without significant degradation of key miRNAs, providing flexibility in handling [20]. However, for other assays, such as INR determination, a needle-to-analysis time of under 5 hours is recommended for robust results [32].
RNA Extraction and RT-qPCR for Plasma miRNA
Materials:
- Qiagen miRNeasy Serum/Plasma Kit (or equivalent) [20]
- DNase/RNase-free reagents
- Synthetic spike-in control (e.g., cel-miR-39-3p) [2]
- RT-qPCR instrumentation and TaqMan MicroRNA Assays [20]
Procedure:
- Spike-in Addition: Thaw plasma/serum aliquots on ice. Add a known quantity of a synthetic non-human miRNA (e.g., cel-miR-39-3p) to each sample prior to RNA extraction. This controls for variations in RNA extraction efficiency and qPCR inhibition [2].
- RNA Extraction: Isolate total RNA using a dedicated kit for biofluids (e.g., Qiagen miRNeasy Serum/Plasma Kit), following the manufacturer's protocol. Elute RNA in a small volume (e.g., 14-28 µL) of nuclease-free water to maximize concentration [20].
- Reverse Transcription (RT): Synthesize cDNA using a High-Capacity RNA-to-cDNA kit. Use a fixed volume of extracted RNA for each reaction to maintain consistency.
- Quantitative PCR (qPCR):
Data Normalization and Analysis:
- Calibration: Use the Cq values of the cel-miR-39-3p spike-in to calibrate and correct for technical variance across samples [2].
- Normalization: Normalize the calibrated Cq values of target miRNAs using a stable endogenous control miRNA identified in your sample set (e.g., miR-16-5p is commonly used) [2]. The relative quantification can then be calculated using the 2^(-ΔΔCq) method.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagent Solutions for Plasma miRNA Analysis via RT-qPCR
| Item | Function / Application | Example Product / Note |
|---|---|---|
| K₂EDTA Tubes | Preferred blood collection for plasma; prevents coagulation. | BD Vacutainer K₂E Tubes (lavender top). |
| RNA Isolation Kit | Purification of small RNAs from plasma/serum. | Qiagen miRNeasy Serum/Plasma Kit [20]. |
| Spike-in Control | Controls for technical variation during RNA extraction and RT-qPCR. | cel-miR-39-3p (synthetic C. elegans miRNA) [2]. |
| RT-qPCR Assays | Sensitive and specific detection of mature miRNAs. | TaqMan MicroRNA Assays (Thermo Fisher) [20]. |
| Nuclease-free Water | Solvent for RNA elution and preparing reagents; ensures no RNase contamination. | -- |
Workflow and Pathway Diagrams
Plasma vs Serum Processing
miRNA Stability & Analysis
Ribonucleic acid (RNA) isolation is a foundational step in molecular biology, with its quality and purity being paramount for downstream applications such as reverse-transcription quantitative polymerase chain reaction (RT-qPCR). This is particularly critical for microRNA (miRNA) quantification in plasma, where the analytes are of low abundance and susceptible to degradation [33] [34]. The choice between traditional phenol-chloroform extraction and various commercial kit-based methods represents a significant practical decision for researchers, balancing factors such as yield, purity, cost, and time [35]. This Application Note provides a structured comparison of these methods and detailed protocols, framed within the context of optimizing RT-qPCR for plasma miRNA research, to guide scientists and drug development professionals in selecting and implementing the most appropriate RNA isolation strategy.
Comparative Analysis of RNA Isolation Methods
Core Principles and Workflow Selection
The primary methods for RNA isolation operate on distinct principles. The organic extraction method, often considered a gold standard, uses a phenol-chloroform mixture to denature proteins and separate the sample into organic and aqueous phases, with RNA partitioning into the aqueous phase [36] [35]. Spin column methods utilize a silica membrane in a filter cartridge to bind nucleic acids in the presence of chaotropic salts, which are then washed and eluted [35]. Magnetic particle methods also rely on silica binding but use paramagnetic beads that can be collected with a magnet, facilitating automation and high-throughput processing [35]. The following workflow diagram outlines the decision-making process for selecting an appropriate RNA isolation method based on key experimental requirements.
Quantitative Performance Comparison
The selection of an RNA isolation method significantly impacts the yield, purity, and suitability of the extracted nucleic acids for sensitive downstream applications like RT-qPCR. The following tables summarize key performance metrics from recent studies comparing different methods across various sample types.
Table 1: Performance of RNA Isolation Methods from Blood and Oral Swab Samples (n=25) [33]
| Extraction Method | Sample Type | Average Yield | Purity (A260/A280) | Suitability for Downstream Use |
|---|---|---|---|---|
| Modified Manual AGPC | Blood | Significantly Higher (p<0.0001) | Significantly Lower (p<0.0001) | Lower purity may be unsuitable |
| Modified Manual AGPC | Oral Swab | Significantly Higher (p<0.0001) | Lower vs. QIAamp (p<0.0001) & OxGEn (p<0.001) | Not recommended |
| QIAamp Viral RNA Mini Kit | Blood & Oral Swab | Lower | Higher | Suitable |
| OxGEn RNA Kit | Blood & Oral Swab | Lower | Higher | Suitable |
Table 2: Performance of miRNA Isolation Kits from Human Plasma (200 µL) [34]
| Commercial Kit | miRNA Quality (RIN) | Total miRNA Quantity | Extraction Efficiency (qPCR Cq) | Cost & Practicality |
|---|---|---|---|---|
| miRNeasy Serum/Plasma Kit | Superior (P < 0.005) | Highest | Highest (for miR-24-3p, miR-191-5p, miR-423-5p, miR-484) | Recommended for limited plasma |
| miRNeasy Mini Kit | Not Specified | Not Specified | Not Specified | Requires RNeasy MinElute Cleanup |
| Agilent RNA Isolation Kit | Not Specified | Not Specified | Not Specified | Column-free, alcohol precipitation |
| Absolutely RNA MicroRNA Kit | Not Specified | Not Specified | Not Specified | Includes DNase digestion step |
Table 3: Advantages and Disadvantages of Core RNA Isolation Techniques [35]
| Method | Key Advantages | Key Disadvantages |
|---|---|---|
| Organic (Phenol-Chloroform) | High yield, "gold standard" for purity, low cost per sample, reagents can be prepared locally [33]. | Labor-intensive, use of hazardous chemicals, not amenable to high-throughput, difficult to automate [35]. |
| Spin Column | Simple protocol, convenient kit format, high purity, amenable to medium-throughput, flexible (centrifugation/vacuum) [35]. | Risk of membrane clogging, low yield with incomplete lysis, can be expensive, potential for gDNA contamination [35]. |
| Magnetic Beads | Most amenable to automation and high-throughput, rapid processing, no filter clogging, no organic waste [35]. | Can be laborious manually, viscous samples impede beads, risk of bead carryover, requires special equipment [35]. |
Detailed Experimental Protocols
Modified Manual Acid Guanidinium Thiocyanate-Phenol-Chloroform (AGPC) Extraction
This protocol, adapted from a 2023 study, is a cost-effective method for achieving high RNA yield from blood samples, though purity may be a concern [33].
Reagent Preparation
- Home-Made TRIzol Reagent: Combine 38 mL water-saturated phenol (pH 4.3), 5 mL glycerol, 3.33 mL sodium acetate (pH 5, 3M), 11.82 g guanidinium thiocyanate (0.8 M final), and 7.61 g ammonium thiocyanate (0.4 M final). Add ddH2O to 100 mL and mix until dissolved (30-60 mins) [33].
- 10x RBC Lysis Buffer: Dissolve 89.9 g NH4Cl, 10.0 g KHCO3, and 2.0 mL of 0.5 M EDTA in 800 mL ddH2O. Adjust pH to 7.3 and bring volume to 1 L. Stable for 6 months at 2-8°C [33].
Extraction Procedure
- Lysis: Add 200 µL of whole blood to 925 µL of 1X RBC Lysis Buffer. Incubate for 10 minutes at room temperature [33].
- Centrifugation: Centrifuge at 1,400 rpm for 10 minutes at 25°C. Discard the supernatant [33].
- Repeat Lysis: Add 1,000 µL of 1X RBC Lysis Buffer to the pellet, incubate for 5 minutes at 25°C, and centrifuge at 3,000 rpm for 2 minutes at 25°C [33].
- Wash: Add 1,000 µL of DPBS to the pellet and centrifuge at 3,000 rpm for 2 minutes at 25°C. Discard the supernatant [33].
- Homogenization: Resuspend the cell pellet in 1,200 µL of home-made TRIzol reagent [33].
- Phase Separation: Add 200 µL of chloroform, vortex vigorously for 15 seconds, and centrifuge at 13,000 rpm for 10 minutes at 4°C [33].
- RNA Precipitation: Transfer the upper aqueous phase to a new tube. Add an equal volume of cold isopropanol, mix by inversion, and incubate at -20°C for 30 minutes. Centrifuge at 13,000 rpm for 10 minutes at 4°C to pellet the RNA. Discard the supernatant [33].
- Wash: Add 500 µL of ice-cold 75% ethanol (prepared with RNase-free water) to the pellet, vortex, and let stand for 10 minutes. Centrifuge at 13,000 rpm for 10 minutes at 4°C. Discard the supernatant and air-dry the pellet for 10 minutes [33].
- Elution: Dissolve the RNA pellet in 20 µL of RNase-free water. Quantify the RNA using a spectrophotometer [33].
Protocol for miRNA Extraction from Plasma Using miRNeasy Serum/Plasma Kit
This protocol, based on a 2021 optimization study, is recommended for obtaining high-quality miRNA from limited plasma volumes, crucial for RT-qPCR analysis [34].
Materials
- Plasma Samples: 200 µL of plasma, clarified by centrifugation at 3,000 × g for 5 min at 4°C to remove cryoprecipitate [34].
- Spike-in Control: 5 µL of 5 pM synthetic C. elegans miRNA cel-miR-39-3p [34].
- Key Reagents: QIAzol Lysis Reagent, Chloroform, 100% Ethanol, RWT and RPE Wash Buffers, RNase-free water [34].
- Equipment: Microfuge, RNeasy MinElute spin column [34].
Standard Workflow with Modification
- Lysis: Mix 200 µL of plasma with 1,000 µL (5 volumes) of QIAzol Lysis Reagent. Vortex and incubate for 5 minutes at room temperature [34].
- Spike-in: Add 5 pM of cel-miR-39-3p to the homogenate and mix [34].
- Phase Separation: Add 200 µL of chloroform, vortex vigorously, and incubate for 2-3 minutes at room temperature. Centrifuge at 12,000 × g for 15 minutes at 4°C [34].
- Aqueous Phase Transfer: Carefully transfer the upper aqueous phase (approximately 600 µL) to a new tube without disturbing the interphase [34].
- Binding: Add 900 µL (1.5 volumes) of 100% ethanol to the aqueous phase and mix thoroughly. Transfer the entire mixture (including any precipitate) to an RNeasy MinElute spin column. Centrifuge at 8,000 × g for 15 seconds. Discard the flow-through [34].
- Washing:
- Wash once with 700 µL of Buffer RWT. Centrifuge and discard flow-through [34].
- Wash once with 500 µL of Buffer RPE. Centrifuge and discard flow-through [34].
- Wash once with 500 µL of 80% ethanol. Centrifuge and discard flow-through [34].
- Centrifuge the empty column for 5 minutes at full speed to dry the membrane [34].
- Elution (Modified - Double Elution):
- Place the column in a new 1.5 mL collection tube. Add 14 µL of RNase-free water directly to the center of the membrane.
- Centrifuge at full speed for 1 minute.
- Return the flow-through containing the eluted RNA to the center of the same column and centrifuge at full speed for an additional 1 minute [34].
- This double elution step significantly enhances the final miRNA yield (P < 0.001) compared to a single elution [34].
The entire workflow for plasma miRNA extraction and analysis is summarized below.
The Scientist's Toolkit: Essential Reagents for RNA Isolation
Selecting the appropriate reagents is critical for the success of any RNA isolation procedure. The following table lists key solutions and their functions, particularly in the context of plasma miRNA research.
Table 4: Key Research Reagent Solutions for RNA Isolation
| Reagent / Kit | Primary Function | Application Context |
|---|---|---|
| QIAzol Lysis Reagent | A monophasic solution of phenol and guanidine thiocyanate for effective cell lysis and inhibition of RNases during sample homogenization [34]. | Used in kits like miRNeasy for lysis of diverse samples, including plasma, serum, and cells. |
| Chaotropic Salts | Disrupt hydrogen bonding in water, dehydrate nucleic acids, and promote their binding to silica matrices in spin columns or magnetic beads [35]. | A key component of binding buffers in most commercial kit protocols. |
| Acid-Phenol:Chloroform | Denatures and partitions proteins into the organic phase or interphase, leaving RNA in the aqueous phase (at acidic pH) [36]. | The core of organic extraction methods; pH is critical for RNA/DNA separation. |
| Silica Spin Columns | Solid-phase matrix that selectively binds RNA in the presence of chaotropic salts, allowing for washing and subsequent elution in a small volume [35]. | The basis for numerous commercial kits from Qiagen, Norgen, and others. |
| Silica-Coated Magnetic Beads | Paramagnetic particles that bind RNA, enabling liquid-handling automation for high-throughput isolation without centrifugation [35]. | Used in kits from Zymo Research and others; ideal for automated workflows. |
| cel-miR-39 Spike-in | Synthetic, exogenous miRNA added to samples at the start of extraction to monitor and normalize for extraction efficiency variations [34]. | Critical quality control for plasma/serum miRNA studies; not an endogenous normalizer [25]. |
| miRNeasy Serum/Plasma Kit | Integrated kit optimized for enriching small RNAs from limited volumes of cell-free biofluids [34]. | The preferred method for plasma miRNA studies according to comparative studies [34]. |
| Norgen Total RNA Purification Kit | Spin column kit for purifying total RNA, including miRNA, without phenol, reported to offer high yields and purity at lower cost [37]. | A cost-effective alternative to other commercial kits for various sample types. |
The choice between phenol-chloroform and commercial kit-based RNA isolation methods is multifaceted. The modified manual AGPC method offers a high-yield, cost-effective alternative for resource-limited settings, particularly for blood samples, though its lower purity may compromise some downstream applications [33]. For research focused on plasma miRNA quantification via RT-qPCR, commercial kits like the miRNeasy Serum/Plasma Kit are generally superior, providing higher purity, better reproducibility, and greater convenience [34]. The implementation of protocol modifications, such as a double elution step, can further enhance miRNA yield from these kits. Ultimately, the optimal method depends on a careful balance of experimental priorities, including sample type, required yield and purity, downstream application sensitivity, available budget, and throughput requirements. The protocols and data provided herein serve as a guide for making this critical decision and ensuring the reliability of subsequent RT-qPCR analyses.
The quantification of circulating microRNAs (miRNAs) in plasma holds significant promise as a minimally invasive approach for diagnosing and monitoring human diseases [20]. However, the accuracy of reverse transcription quantitative polymerase chain reaction (RT-qPCR) results is highly dependent on proper data normalization to account for technical variations occurring during sample processing [25] [13]. The implementation of exogenous spike-in controls, specifically Cel-miR-39 from C. elegans, provides a robust strategy for monitoring technical performance and normalizing experimental data in plasma miRNA studies [38] [18].
Cel-miR-39 is ideally suited as a spike-in control because it bears no sequence homology to any known human miRNA, thereby eliminating false positives or cross-reactivity in human samples [18]. When added at the initial stage of RNA extraction, this synthetic miRNA serves as a critical quality control by monitoring extraction efficiency, reverse transcription, and PCR amplification efficiency, ultimately enabling more accurate normalization of target miRNA expression levels [18] [39].
Cel-miR-39 in the miRNA Analysis Workflow
The following diagram illustrates the integration points and dual functionality of cel-miR-39 spike-in control within the plasma miRNA analysis workflow:
Comprehensive Protocol for Cel-miR-39 Implementation
Preparation of Cel-miR-39 Spike-In Solution
The first critical step involves proper preparation of the cel-miR-39 spike-in solution [18]:
- Resuspension: Centrifuge the original tube containing 5 nmol lyophilized cel-miR-39 (e.g., mirVana miRNA mimic Assay ID: MC10956) at 500× g for 1 minute at room temperature. Resuspend in 200 μL of nuclease-free, molecular-biology-grade water to create a 25 μM stock solution.
- Vortexing: Thoroughly vortex the stock solution to achieve a homogenous mixture.
- Working Solution Preparation: Prepare a 0.5 μM working solution by performing a 1:50 dilution in nuclease-free water. Mix thoroughly by vortexing for 30 seconds.
- Concentration Verification: Using 1-10 μL of the 0.5 μM working solution, measure the actual concentration in ng/μL using a Qubit Fluorometer and miRNA Concentration Kit according to the manufacturer's protocol.
- Serial Dilution: Prepare serial (1:10) dilutions from the verified stock. Vortex for 30 seconds, and rest on ice for an additional 30 seconds. Repeat this process three times before transferring the appropriate volume to create a dilution series with concentrations of: 100, 10, 1, 0.1, 0.01, and 0.001 ng/μL.
RNA Extraction with Cel-miR-39 Spike-In
During RNA isolation from plasma or serum samples [18] [39]:
- Add a standardized amount (typically 20 femtomolar) of cel-miR-39 spike-in to 300 μL of plasma or serum during the lysis step of RNA extraction [39].
- Proceed with RNA isolation according to the manufacturer's protocol for your chosen isolation kit (e.g., Qiagen miRNeasy Serum/Plasma Kit or miRCURY RNA Isolation Kit).
- Elute RNA in 30 μL of nuclease-free H2O and store at -80°C until cDNA synthesis.
Reverse Transcription and qPCR Analysis
For cDNA generation and amplification [18]:
- Reverse Transcription: Prepare a cDNA master mix using the TaqMan miRNA Reverse Transcription Kit with the following components per reaction: 0.15 μL of dNTP Mix, 0.19 μL of RNase Inhibitor, 1.5 μL of 10X RT Buffer, 1 μL of Multiscribe Reverse Transcriptase, 0.75 μL of miR-39 RT Primers (20×), and 10.41 μL of nuclease-free water.
- Add 14 μL of the master mix to all tubes, followed by 1 μL of RNA template.
- Run the thermocycler with the following conditions: 5 minutes at 4°C, 30 minutes at 16°C, 30 minutes at 42°C, 5 minutes at 85°C, and hold at 4°C indefinitely with the lid temperature set to 105°C.
- qPCR Analysis: Prepare a ddPCR or qPCR master mix containing: 12.5 μL of ddPCR Supermix (no dUTP), 1.25 μL of miR-39 VIC-labeled Probe (20×), and 6.25 μL of nuclease-free water per reaction.
- Add 5 μL of cDNA to each reaction and perform droplet generation or standard qPCR amplification.
Performance Assessment and Normalization Strategies
Monitoring Technical Performance
The recovery efficiency of cel-miR-39 should be consistent across samples. Studies recommend setting acceptable threshold values for cel-miR-39 Cq values. For instance, one methodological study repeated RNA isolation when the Cq value of spike-in cel-miR-39-3p was >30 [39]. Consistent Cq values across samples indicate minimal technical variability in RNA extraction and reverse transcription efficiency.
Data Normalization Approaches
Cel-miR-39 can be utilized in different normalization strategies:
Table 1: Normalization Strategies Using Cel-miR-39
| Normalization Approach | Implementation | Application Context |
|---|---|---|
| Single Spike-In Normalization | Normalize target miRNA expression to cel-miR-39 alone using the 2−ΔΔCq method [19] | Studies with limited sample volume or when endogenous controls are unstable |
| Combined Normalization | Use the average of cel-miR-39 and a stable endogenous miRNA (e.g., miR-16-5p) [39] | When validated endogenous controls are available; provides more robust normalization |
| Absolute Quantification | Use cel-miR-39 of known concentration to generate standard curves for absolute quantification [18] | When copy number determination is required rather than relative expression |
Research indicates that optimal normalization is often achieved using combined approaches. One study evaluating methodological challenges found that the averaged detection values of spike-in cel-miR-39-3p and endogenous miR-16-5p provided superior normalization compared to either alone [39].
Research Reagent Solutions
Table 2: Essential Materials and Research Reagents for Cel-miR-39 Implementation
| Reagent/Kit | Manufacturer/Supplier | Function in Protocol |
|---|---|---|
| microRNA (cel-miR-39) Spike-In Kit | Norgen Biotek [38] | Provides quantified synthetic cel-miR-39 for spike-in during RNA extraction |
| miRNeasy Serum/Plasma Kit | Qiagen [20] | RNA isolation from plasma/serum with optimal recovery of small RNAs |
| TaqMan miRNA Reverse Transcription Kit | Applied Biosystems [18] | cDNA synthesis from miRNA templates including spike-in controls |
| miRcury LNA Universal RT microRNA PCR Kit | Exiqon [39] | Alternative system for miRNA detection with SYBR Green chemistry |
| Droplet Digital PCR Supermix | Bio-Rad [18] | Enables absolute quantification of miRNA copies using droplet digital PCR |
| miRNA Concentration Assay Kit | Thermo Fisher (Qubit) [18] | Accurate quantification of miRNA working solutions prior to use |
Troubleshooting and Technical Considerations
Addressing Common Implementation Challenges
Several factors can affect the performance of cel-miR-39 spike-in controls:
- Inconsistent Spike-In Recovery: Large variations in cel-miR-39 Cq values across samples may indicate technical issues in RNA extraction. Ensure consistent pipetting technique and thorough mixing after spike-in addition.
- Hemolysis Interference: Hemolysis can significantly affect endogenous miRNA levels but should not impact cel-miR-39 recovery. Monitor hemolysis using absorbance measurements at 414 nm [13] or the miR-23a-3p/miR-451a ratio [13].
- Inhibition in Reverse Transcription: If cel-miR-39 Cq values are abnormally high, check for reverse transcription inhibitors. Diluting RNA samples or additional purification may be necessary.
Limitations and Complementary Approaches
While cel-miR-39 is valuable for monitoring technical variability, it cannot account for biological variations in sample composition. Some researchers note that cel-miR-39 "cannot be considered the best candidate for use as a normalizer; instead, it should be used as a quality control for miRNA extraction" [25]. For comprehensive normalization, consider combining cel-miR-39 with validated endogenous controls that demonstrate stable expression across your experimental conditions [25] [39].
The implementation of cel-miR-39 spike-in controls represents a critical quality assurance measure in plasma miRNA quantification studies. When properly integrated into the analytical workflow as described in this protocol, cel-miR-39 enables researchers to monitor technical performance, normalize experimental data, and generate more reliable and reproducible results. This standardization is essential for advancing circulating miRNAs as clinically useful biomarkers across various disease contexts, including cancer, cardiovascular conditions, and infectious diseases like COVID-19 [20] [25].
Quantitative reverse-transcription PCR (RT-qPCR) is a powerful tool for gene expression analysis, but the detection of microRNAs (miRNAs) presents a unique challenge due to their short length (typically 17-24 nucleotides) [40]. Standard RT-qPCR methods require a template that is at least twice the length of the PCR primers, making miRNAs too short for conventional approaches [40]. Stem-loop reverse transcription primers provide an elegant solution to this problem, enabling specific and sensitive quantification of miRNA expression levels [40] [41]. This application note details the optimized protocols and experimental considerations for implementing stem-loop RT-qPCR in plasma miRNA research, with particular relevance to biomarker discovery and drug development applications.
The core innovation of stem-loop primers lies in their highly stable stem-loop structure that extends the length of the cDNA during reverse transcription, thereby creating a sufficiently long template for subsequent qPCR amplification [40]. This approach incorporates multiple design features that collectively enhance assay performance: the stem-loop RT primer lengthens the cDNA target, the forward PCR primer adds nucleotides to optimize melting temperature, the reverse primer disrupts the stem-loop structure, and the hydrolysis probe spans much of the original miRNA sequence with a minor groove binding (MGB) moiety to enhance specificity [40]. When properly optimized, this method offers exceptional specificity and sensitivity, enabling detection of miRNAs from minimal RNA input, with some protocols requiring as little as 1-10 pg of total RNA [41].
For researchers investigating circulating biomarkers, plasma miRNAs offer significant promise but also present technical challenges. miRNAs demonstrate remarkable stability in plasma and serum, maintaining consistent Cq values for up to 24 hours at room temperature, which enables feasible handling in routine clinical settings [20]. This stability, combined with their disease-specific expression patterns, makes miRNAs attractive candidates for diagnostic biomarkers across various conditions including cancer, autoimmune disorders, and cardiovascular diseases [20]. The protocols outlined herein provide a framework for reliable miRNA quantification in plasma samples to support these research applications.
Core Principles and Methodologies
Stem-Loop Primer Design and Mechanism
Stem-loop primers employ a sophisticated architectural design that enables precise miRNA quantification. The primer consists of three critical regions: a complementary sequence that binds specifically to the 3' end of the mature miRNA target, a stem structure formed by self-complementary sequences, and a loop region that connects the stem elements [40]. This configuration creates a geometrically constrained structure that enhances binding specificity, particularly for the 3' region of the miRNA, which is the most variable portion of the molecule and therefore provides the greatest discriminatory power between closely related miRNA family members.
During reverse transcription, the stem-loop primer binds to the mature miRNA and is extended to create a cDNA product that incorporates the complementary miRNA sequence along with the extended stem-loop structure. This elongated cDNA serves as an optimal template for subsequent qPCR amplification. The forward PCR primer is designed with two segments: a portion that binds to the original miRNA sequence and an extension that adds length to optimize melting temperature and enhance assay specificity [40]. The reverse primer targets the stem-loop sequence, effectively disrupting the secondary structure during amplification. For detection, a sequence-specific hydrolysis probe with a minor groove binding (MGB) moiety is typically employed, with the probe positioned over much of the original miRNA sequence to maximize specificity [40].
Recent advancements in stem-loop primer technology include the development of chimeric dU stem-loop primers that incorporate a deoxyuracil (dU) base near the RNA matching region [41]. Following reverse transcription, uracil-DNA glycosylase (UDG) treatment removes the dU base and disrupts the stem-loop structure of the RT product, reducing potential interference from base stacking and steric hindrance during amplification [41]. This innovation has demonstrated 1.1- to 3.4-fold improvements in detection sensitivity and specificity compared to traditional stem-loop primers, particularly beneficial when working with limited sample material [41].
Comparative Analysis of Primer Technologies
Table 1: Comparison of Primer Technologies for miRNA Quantification
| Primer Type | Mechanism of Action | Advantages | Limitations | Optimal Use Cases |
|---|---|---|---|---|
| Stem-Loop | Uses structured primer to extend cDNA; adds sequence for PCR priming [40] | High specificity and sensitivity [40]; Discriminates closely related miRNAs [40] | Complex primer design; Requires optimization | Absolute quantification; Low-abundance miRNAs |
| Linear | Simple linear primer binds miRNA for reverse transcription | Simple design; Easy to implement | Lower specificity; May detect pre-miRNAs | High abundance targets; Preliminary screening |
| Chimeric dU Stem-Loop | Incorporates dU bases; UDG treatment post-RT destroys stem-loop [41] | Reduced steric hindrance [41]; Enhanced sensitivity (1.1-3.4×) [41] | Additional enzymatic step; More complex workflow | Trace samples; Bacterial sRNAs; Maximum sensitivity |
Workflow Visualization
The following diagram illustrates the complete workflow for stem-loop RT-qPCR, from sample preparation through data analysis:
Experimental Protocols
Plasma Sample Collection and RNA Extraction
Proper sample collection and processing are critical for reliable plasma miRNA analysis. Blood should be collected in K₂EDTA tubes (for plasma) or clotting tubes (for serum) [20]. For plasma preparation, centrifuge blood samples at 1200×g for 10 minutes at room temperature, followed by careful collection of the top layer and a secondary centrifugation at 1500×g for 5 minutes to remove residual cells [20]. Aliquot plasma/serum samples (0.5 mL recommended) and store at -80°C if not processed immediately.
For RNA extraction, use specialized kits designed for low-abundance RNAs from serum or plasma, such as the Qiagen miRNeasy Serum/Plasma Kit [20]. Incorporate a synthetic spike-in control (e.g., cel-miR-39) during extraction to monitor efficiency and potential inhibitors [25]. Modify elution conditions by increasing centrifugation time to 2 minutes and using 28 μL of nuclease-free water instead of the recommended 14 μL to enhance yield [20]. The quality of extracted RNA can be assessed via UV spectrophotometry, though the low concentration may challenge conventional methods.
Stem-Loop Reverse Transcription Protocol
The reverse transcription reaction converts miRNAs to extended cDNA templates suitable for qPCR amplification.
Table 2: Reverse Transcription Reaction Setup
| Component | Volume | Final Concentration | Purpose |
|---|---|---|---|
| Total RNA (or 1-10 ng plasma RNA) | Variable | - | Sample input |
| Stem-Loop RT Primer (1 μM) | 1 μL | 50 nM | miRNA-specific reverse transcription |
| dNTP Mix (10 mM) | 0.5 μL | 500 μM | cDNA synthesis |
| Reverse Transcriptase | 1 μL | - | Enzyme catalyst |
| RT Buffer (10X) | 1 μL | 1X | Reaction conditions |
| RNase Inhibitor | 0.5 μL | - | RNA protection |
| Nuclease-free Water | To 10 μL | - | Volume adjustment |
Execute the following thermal cycling conditions: incubate at 16°C for 30 minutes (primer annealing), 42°C for 30-60 minutes (reverse transcription), 85°C for 5 minutes (enzyme inactivation), then hold at 4°C [40]. For chimeric dU stem-loop primers, include an additional step: treat with uracil-DNA glycosylase (UDG) according to manufacturer specifications to disrupt the stem-loop structure before qPCR [41].
Quantitative PCR Amplification
The qPCR step amplifies and detects specific miRNA targets with high sensitivity.
Table 3: qPCR Reaction Components
| Component | Volume | Final Concentration | Purpose |
|---|---|---|---|
| cDNA Template | 2 μL | - | Amplification target |
| Forward Primer (10 μM) | 0.5 μL | 250 nM | Target-specific forward priming |
| Reverse Primer (10 μM) | 0.5 μL | 250 nM | Universal reverse priming |
| TaqMan Probe or SYBR Green Master Mix | 10 μL | 1X | Detection chemistry |
| Nuclease-free Water | 7 μL | - | Volume adjustment |
| Total Volume | 20 μL | - | - |
Program the thermal cycler with an initial denaturation at 95°C for 10 minutes, followed by 40 cycles of 94°C for 15 seconds and 60°C for 1 minute [19]. Include appropriate controls: no-template controls (NTC) to detect contamination, and inter-plate calibrators to normalize between runs. Perform technical replicates (at least triplicate) for each biological sample to ensure statistical robustness.
Data Analysis and Normalization
Quantification Methods and Efficiency Calculation
Accurate quantification of RT-qPCR data requires appropriate mathematical models. The two primary methods for relative quantification are the Livak method (2^(-ΔΔCT)) and the Pfaffl method [42] [43]. The Livak method assumes ideal amplification efficiency (100%) for both target and reference genes and calculates fold change using the formula: FC = 2^(-ΔΔCT), where ΔΔCT = (CTtarget - CTreference)treatment - (CTtarget - CTreference)control [42] [43]. This method is simple but depends on nearly perfect amplification efficiency.
The Pfaffl method provides a more flexible approach by accounting for differences in amplification efficiencies between target and reference genes [42]. The formula is: FC = (Etarget)^(-ΔCTtarget) / (Ereference)^(-ΔCTreference), where E represents amplification efficiency [42]. This method is more accurate when amplification efficiencies differ from 100% or vary between genes.
PCR efficiency should be calculated using a standard curve with serial dilutions (e.g., 1:10, 1:100, 1:1000, 1:10000) [43]. Plot the log(10) dilution factor against the CT values and calculate the slope. Efficiency is then determined as: Efficiency (%) = (10^(-1/slope) - 1) × 100 [43]. Acceptable efficiency ranges from 85-110% [43]. The rtpcr package in R provides a comprehensive toolset for these calculations, accommodating efficiency values and multiple reference genes [42].
Normalization Strategies for Plasma miRNAs
Proper normalization is essential for accurate miRNA quantification. For plasma samples, both endogenous and exogenous normalization approaches are used. The synthetic spike-in control cel-miR-39 is commonly added during RNA extraction to monitor technical variability, but as an exogenous control, it cannot account for biological variation [25]. Endogenous normalizers should exhibit stable expression across experimental conditions.
Selection of appropriate reference miRNAs requires empirical validation. A recent COVID-19 study identified hsa-miR-205-3p as a stable normalizer in plasma through RNA sequencing, establishing selection criteria including fold regulation = 1, p-value > 0.990, and discovery date [25]. Commonly used references like miR-16 and miR-191 may not be stable in all contexts; for example, miR-16-5p shows differential expression in COVID-19 patients potentially due to binding sites in the viral genome [25].
Researchers should validate potential normalizers in their specific experimental system by comparing expression stability across all sample groups using algorithms like RefFinder [25]. When no single gene shows sufficient stability, a geometric mean of multiple reference genes may provide more robust normalization.
The Scientist's Toolkit
Essential Research Reagent Solutions
Table 4: Key Reagents for Stem-Loop RT-qPCR
| Reagent/Catalog Number | Manufacturer | Primary Function | Application Notes |
|---|---|---|---|
| miRNeasy Serum/Plasma Kit (217184) | Qiagen | Total RNA extraction from plasma/serum | Includes cel-miR-39 spike-in; Modified elution improves yield [20] |
| TaqMan MicroRNA Assays (4427975) | Thermo Fisher | Sequence-specific detection | Includes stem-loop RT primers, PCR primers, and probes [20] |
| PrimeScript RT Reagent Kit | Takara Bio | Reverse transcription | Compatible with stem-loop primers [19] |
| SYBR Premix Ex Taq | Takara | qPCR amplification | For SYBR Green-based detection [19] |
| High-Capacity RNA-to-cDNA Kit (01127021) | Applied Biosystems | Reverse transcription | Alternative for cDNA synthesis [20] |
Troubleshooting Common Challenges
Several technical challenges may arise when implementing stem-loop RT-qPCR. Poor amplification efficiency may result from suboptimal primer design, inhibitor carryover, or inadequate RNA quality. To address this, verify primer specificity, include purification steps, and check RNA integrity. For high background signal, optimize primer concentrations, increase annealing temperature, or include additional controls to identify primer-dimer formation. Variable Cq values across replicates may stem from pipetting inaccuracies, insufficient mixing, or template degradation; use calibrated pipettes, mix reactions thoroughly, and ensure proper sample storage.
When analyzing plasma samples, hemolysis represents a particular concern as it releases cellular miRNAs that can alter the plasma miRNA profile [20]. Visually inspect samples for pink discoloration indicating hemoglobin contamination, and consider measuring specific hemolysis indicators such as miR-451a levels, which elevate in hemolyzed samples. Additionally, pre-analytical variables including processing delays, storage conditions, and freeze-thaw cycles can impact miRNA stability, though studies demonstrate remarkable stability of many miRNAs for up to 24 hours at room temperature [20].
Stem-loop RT-qPCR provides a robust methodology for sensitive and specific quantification of miRNAs in plasma samples. The strategic primer design enables detection of these short nucleic acid sequences that would otherwise be inaccessible to conventional RT-qPCR approaches. Recent innovations such as chimeric dU stem-loop primers with UDG treatment further enhance sensitivity and specificity, pushing detection limits to minimal RNA inputs [41]. When implementing these protocols, careful attention to normalization strategies using validated reference genes or synthetic controls is essential for biologically meaningful results [25].
For researchers in biomarker discovery and drug development, plasma miRNA profiling offers significant potential for non-invasive diagnostic applications across diverse disease areas including cancer, metabolic disorders, and infectious diseases [20]. The protocols outlined in this application note provide a foundation for reliable miRNA quantification, with particular emphasis on handling variables unique to plasma samples. As the field advances, standardization of pre-analytical procedures and normalization approaches will further enhance the reproducibility and clinical utility of plasma miRNA measurements.
The quantification of microRNAs (miRNAs) in plasma presents unique challenges, including low abundance of targets, the presence of inhibitors, and the need to distinguish between highly similar miRNA isoforms (isomiRs). Reverse Transcription quantitative PCR (RT-qPCR) is a widely adopted method for this purpose due to its sensitivity, specificity, and quantitative capabilities [27] [44]. This application note provides a detailed protocol for setting up a RT-qPCR assay optimized for the accurate quantification of miRNAs in plasma, a critical requirement for biomarker discovery and diagnostic assay development in drug research.
Assay Selection and Design
Selecting the appropriate assay is the most critical step for the specific detection of plasma miRNAs. The choice hinges on the need to discriminate the target miRNA from closely related sequences and isomiRs.
2.1 Stem-Loop RT-qPCR Assay For superior specificity and sensitivity, the stem-loop RT-qPCR method is recommended. This assay uses a stem-loop reverse transcription primer that binds to the 3' end of the miRNA. The design confers enhanced specificity due to its structural constraints and base stacking, increasing the sensitivity for detecting low-abundance miRNAs from minimal RNA input [45]. The process involves two key steps:
- Reverse Transcription: A miRNA-specific stem-loop primer is hybridized and reverse transcribed.
- qPCR Amplification: The cDNA is amplified using a miRNA-specific forward primer and a universal reverse primer [45].
2.2 Polyadenylation-Tailed RT-qPCR Assay An alternative sensitive and cost-effective method involves polyadenylating the miRNA prior to reverse transcription using poly(A) polymerase. The cDNA is then synthesized using an anchored oligo(dT) primer, and amplification is performed with a miRNA-specific forward primer and a universal reverse primer [44]. This method is particularly advantageous for detecting specific isomiRs that may be more abundant than their canonical counterparts in plasma [44].
Table 1: Comparison of miRNA RT-qPCR Assay Types
| Assay Type | Principle | Advantages | Disadvantages | Best For |
|---|---|---|---|---|
| Stem-Loop RT-qPCR [45] | Sequence-specific stem-loop RT primer and a universal reverse primer. | High specificity and sensitivity; Detects miRNAs from as little as 20 pg total RNA. | Requires careful design of stem-loop primers. | High-sensitivity quantification of specific miRNA targets. |
| Polyadenylation-Tailed RT-qPCR [44] | Polyadenylation of miRNA followed by RT with anchored oligo(dT) and qPCR with a specific forward primer. | Cost-effective (SYBR Green chemistry); No need for proprietary kits; Excellent for isomiRs. | Requires polyadenylation step. | Discriminating and quantifying specific isomiR variants. |
The following diagram illustrates the workflow and key decision points for establishing a robust miRNA RT-qPCR assay:
Detailed Experimental Protocol
Protocol A: Stem-Loop RT-qPCR for miRNA
Principles: This protocol [45] utilizes a stem-loop reverse transcription primer for highly specific cDNA synthesis, ideal for quantifying specific miRNAs from low-abundance samples like plasma.
Required Materials:
- Purified total RNA from plasma.
- Stem-loop RT primer (designed per Table 1 in [45]).
- miRNA-specific forward primer, universal reverse primer.
- Reverse transcriptase (e.g., PrimeScript RT Enzyme Mix).
- RNase-free water.
- SYBR Green I PCR Master Mix or equivalent.
- Real-time PCR instrument.
Procedure:
- Reverse Transcription (RT) Reaction:
- Prepare RT mix on ice: 7 μL RNA template, 1 μL stem-loop RT primer (1 μM), 4 μL 5x RT buffer, 2 μL dNTP mix (10 mM each), 0.5 μL RNase inhibitor, 1 μL reverse transcriptase, 4.5 μL RNase-free water. Total volume: 20 μL.
- Incubate in a thermocycler: 65°C for 5 min (to denature secondary structures), hold on 4°C for primer annealing. Then, 42°C for 60 min (cDNA synthesis), 70°C for 15 min (enzyme inactivation). Hold at 4°C.
qPCR Reaction Setup:
- Prepare qPCR master mix on ice: 10 μL SYBR Green I PCR Master Mix (2x), 3 μL miRNA-specific forward primer (5 μM), 1.4 μL universal reverse primer (5 μM), 2 μL diluted (1:10) cDNA, 3.6 μL RNase-free water. Total volume: 20 μL [46].
- Run reactions in triplicate. Include no-template controls (NTC) and no-RT controls.
Thermal Cycling:
- Use the following conditions: 95°C for 5 min (initial denaturation); 40 cycles of 95°C for 15 s (denaturation) and 60°C for 60 s (annealing/extension) [46].
- Perform melt curve analysis post-amplification to verify product specificity.
Protocol B: Polyadenylation-Tailed RT-qPCR for IsomiRs
Principles: This protocol [44] includes a polyadenylation step to tail miRNAs, enabling reverse transcription with a universal anchored oligo(dT) primer. It is excellent for discriminating and quantifying specific isomiR variants using SYBR Green chemistry.
Required Materials:
- Purified total RNA from plasma.
- Poly(A) Polymerase.
- Anchored oligo(dT) RT primer (e.g., 5'-...VN-3', where V is A/G/C).
- miRNA-specific forward and universal reverse qPCR primers.
- Reverse transcriptase, dNTPs.
- SYBR Green I PCR Master Mix.
- Real-time PCR instrument.
Procedure:
- Polyadenylation Reaction:
- Prepare reaction mix: 1-10 μL RNA, 1 μL Poly(A) Polymerase buffer (10x), 1 μL ATP (10 mM), 0.5 μL Poly(A) Polymerase, add RNase-free water to 10 μL.
- Incubate at 37°C for 30-60 min. Enzyme can be heat-inactivated if required.
Reverse Transcription (RT):
- Add to the polyadenylation reaction: 4 μL 5x RT buffer, 1 μL anchored oligo(dT) primer (10 μM), 2 μL dNTP mix (10 mM each), 0.5 μL RNase inhibitor, 1 μL reverse transcriptase, 1.5 μL RNase-free water. Total volume: 20 μL.
- Incubate in a thermocycler: 42°C for 60 min, followed by 70°C for 15 min. Hold at 4°C.
qPCR Reaction Setup and Thermal Cycling:
- Prepare qPCR mix as in Protocol A, Section 3.1, using primers specific for the target isomiR.
- Use the same thermal cycling conditions and melt curve analysis as in Protocol A, Section 3.1.
Master Mix Preparation and Cycling Conditions
4.1 Master Mix Preparation For consistent results, prepare a master mix for all technical replicates to minimize pipetting error. The following table provides a generalized guide for a single 20 μL SYBR Green-based qPCR reaction. Optimization of primer concentration may be necessary [47].
Table 2: qPCR Master Mix Components for a Single 20 μL Reaction
| Component | Final Concentration | Volume per Reaction (μL) | Function |
|---|---|---|---|
| SYBR Green I PCR Master Mix (2X) | 1X | 10 | Contains Hot Start DNA polymerase, dNTPs, Mg²⁺, and fluorescent dye |
| Forward Primer (5 μM) | 300-900 nM [47] | 1.2 - 3.6 (e.g., 3.0) | Binds specifically to the target cDNA strand |
| Reverse Primer (5 μM) | 300-900 nM [47] | 1.2 - 3.6 (e.g., 1.4) | Binds specifically to the complementary cDNA strand |
| cDNA Template | - | 2 | The amplified target |
| RNase-free Water | - | To 20 μL | Adjusts final volume |
4.2 Optimized Thermal Cycling Conditions Standardized cycling conditions ensure efficient amplification. The following protocol is suitable for most applications but can be adjusted for specific master mixes or difficult templates.
Table 3: Standard Thermal Cycling Protocol for miRNA qPCR
| Stage | Cycles | Temperature | Time | Purpose |
|---|---|---|---|---|
| Initial Denaturation | 1 | 95°C | 5 min | Activates polymerase, denatures cDNA |
| Amplification | 40-45 | 95°C | 15 s | Denatures double-stranded DNA |
| 55-65°C* | 30-60 s | Primer annealing | ||
| 72°C | 30 s | Primer extension | ||
| Melt Curve Analysis* | 1 | 65°C to 95°C, increment 0.5°C | 5 s/step | Verifies amplification specificity |
*The annealing temperature should be optimized for each primer pair, with 60°C being a common starting point [46] [47]. Extension time can be adjusted based on amplicon length and polymerase speed; for short miRNA amplicons (<150 bp), a combined annealing/extension step at 60°C is often sufficient [46]. *Required for SYBR Green assays.
The Scientist's Toolkit: Essential Reagents and Materials
Table 4: Key Research Reagent Solutions for miRNA RT-qPCR
| Reagent / Kit | Function / Application | Example Notes |
|---|---|---|
| Stem-Loop Primers [45] | Reverse transcription of specific miRNAs. | The 3' 6-8 nucleotides are reverse-complementary to the target miRNA; confers high specificity. |
| SYBR Green I Master Mix [46] [44] | Fluorescent detection of double-stranded DNA during qPCR. | Cost-effective; requires melt curve analysis to confirm specificity. |
| TaqMan Probe Master Mix | Sequence-specific fluorescent detection during qPCR. | Higher specificity than SYBR Green; requires a separate probe for each miRNA [45]. |
| Poly(A) Polymerase [44] | Adds a poly(A) tail to miRNAs for universal priming. | Essential for the polyadenylation-tailed RT-qPCR protocol. |
| Anchored Oligo(dT) Primer [44] | Reverse transcription of polyadenylated miRNAs. | The "anchor" (V; A/G/C) ensures priming at the start of the poly(A) tail. |
| WarmStart/HotStart DNA Polymerase [47] | Polymerase for qPCR; reduces non-specific amplification. | Improves assay specificity and sensitivity. |
| DNase I (RNase-free) [48] | Degrades contaminating genomic DNA in RNA samples. | Critical when primers are not designed to span exon-exon junctions. |
Data Analysis and QC
Proper data analysis is crucial for accurate interpretation. The following workflow outlines the key steps, from raw data acquisition to final relative quantification.
- Cycle Threshold (Ct) and QC: The Ct is the cycle number at which the fluorescence crosses a threshold set above the baseline [49]. Check amplification plots and melt curves for single, specific products [46] [44].
- PCR Efficiency Calibration: Amplification efficiency should be 90-110%, with a standard curve R² ≥ 0.99 [47] [49]. Calculate efficiency using a dilution series: Efficiency (%) = (10^(–1/slope) – 1) × 100 [49].
- Relative Quantification: Use the 2^–ΔΔCt method to calculate fold-change in gene expression relative to a calibrator sample (e.g., healthy control), normalized to a stable reference gene [49]. Ensure the reference gene is validated for your specific plasma samples and experimental conditions [50].
The quantification of circulating microRNAs (miRNAs) in plasma has emerged as a promising approach for non-invasive biomarker development in various diseases, including cancer, cardiovascular conditions, and metabolic disorders. While quantitative Real-Time PCR (RT-qPCR) has been the gold standard for miRNA expression analysis, this technique faces significant challenges including normalization issues, dependence on external calibrators, and sensitivity limitations when dealing with low-abundance targets in complex biological fluids [51] [52]. Digital PCR (dPCR) represents a transformative technological advancement that enables absolute quantification of nucleic acids without standard curves, offering superior precision, accuracy, and reproducibility for plasma miRNA analysis [53] [52].
This Application Note provides detailed methodologies for implementing dPCR in plasma miRNA quantification workflows, highlighting key advantages over traditional RT-qPCR approaches and presenting experimental data demonstrating its enhanced performance characteristics for biomarker development and validation.
Comparative Analysis: dPCR vs. RT-qPCR for miRNA Quantification
Technical Foundations and Advantages
Digital PCR operates through sample partitioning into thousands of nanoliter-scale reactions, with endpoint detection that allows absolute quantification based on Poisson statistics [54]. This fundamental difference in methodology provides several distinct advantages for miRNA quantification:
- Absolute quantification without requirement for standard curves or reference genes [52] [54]
- Enhanced resistance to PCR inhibitors present in plasma samples due to sample partitioning [51] [54]
- Superior precision for detecting low-fold changes and rare targets [52] [54]
- Higher tolerance to amplification efficiency variations as data collection occurs at endpoint rather than during exponential phase [54]
Performance Comparison in Plasma miRNA Studies
Table 1: Comparative Performance of dPCR and RT-qPCR in miRNA Quantification
| Parameter | RT-qPCR | Digital PCR | Experimental Evidence |
|---|---|---|---|
| Quantification Method | Relative (requires normalization) or absolute with standard curve | Absolute without standard curve | [52] [54] |
| Precision (CV) | Higher variability, especially for low-abundance targets | Significantly improved precision (CV for let-7a: p=0.028) | [52] |
| Sensitivity | Limited for targets <1% mutation rate | Detects mutation rates ≥0.1% | [54] |
| Dynamic Range | Broad dynamic range | Optimal for precise quantification | [54] |
| Normalization | Requires reference genes with lack of consensus | Reduced normalization needs | [51] [52] |
| Inhibitor Tolerance | Affected by plasma inhibitors | Higher tolerance due to partitioning | [51] [54] |
Experimental Protocols for Plasma miRNA Quantification Using dPCR
Sample Collection and Processing
Proper sample collection and processing are critical for reliable plasma miRNA quantification:
- Collect peripheral blood in EDTA-coated tubes (e.g., Vacutainer Systems, Becton Dickinson) [51]
- Centrifuge at 1200-3000× g for 10 minutes at 4°C within 30 minutes post-collection [51] [20]
- Transfer plasma to fresh tubes and perform secondary centrifugation at 1500× g for 5 minutes to remove residual cells [20]
- Aliquot processed plasma and store at -80°C until analysis [51] [18]
- Avoid freeze-thaw cycles to maintain miRNA integrity [18]
RNA Extraction and Alternative Extraction-Free Protocol
Conventional RNA Extraction
- Extract total RNA from 200 μL plasma using specialized kits (e.g., miRNeasy Serum/Plasma Kit, Qiagen) [53] [3]
- Elute RNA in 20-50 μL nuclease-free water [51] [53]
- Quantify extraction yield using fluorometric methods (e.g., Qubit miRNA Assay Kit) when possible [18]
Extraction-Free Protocol (Direct Lysate Analysis)
Emerging protocols eliminate RNA extraction, minimizing loss and variability:
- Incubate 200 μL plasma with proteinase K (200 μg/mL final concentration) for 15 minutes at room temperature with occasional agitation [51]
- Heat samples at 75°C for 5 minutes with agitation to inactivate enzymes [51]
- Proceed directly to reverse transcription without RNA purification [51]
This extraction-free approach has demonstrated high accuracy in detecting low-copy-number and highly expressed miRNAs in human plasma, significantly streamlining the workflow [51].
Reverse Transcription and Preamplification
- Use the TaqMan Advanced miRNA cDNA Synthesis Kit (Thermo Fisher Scientific) according to manufacturer's instructions [51] [53]
- Perform poly(A) tailing at 37°C for 45 minutes followed by adapter ligation [51] [3]
- Conduct reverse transcription using universal RT primers at 42°C for 15 minutes [51]
- Include preamplification (14 cycles) to enrich target sequences [51]
- For multiplex assays, optimize primer concentrations to maintain amplification efficiency [53]
Digital PCR Setup and Analysis
Chip-Based dPCR (QuantStudio 3D System)
- Prepare dPCR reaction mix containing cDNA, TaqMan assays, and dPCR master mix [51]
- Load samples onto chips using specialized loader [51]
- Perform thermocycling: enzyme activation at 96°C for 10 minutes, followed by 40 cycles of denaturation at 98°C for 30 seconds and annealing/extension at 56°C for 2 minutes [51]
- Analyze using QuantStudio 3D AnalysisSuite with Poisson correction [51]
Droplet Digital PCR (ddPCR System)
- Prepare 25 μL reactions containing ddPCR supermix, TaqMan assays, and cDNA template [18] [52]
- Generate droplets using droplet generator (DG8 Cartridges, Bio-Rad) [18]
- Transfer 40 μL droplets to 96-well plate and seal with pierceable foil [18]
- Amplify with similar thermal profile to chip-based systems [52]
- Read droplets using droplet reader and analyze with companion software [18]
Normalization Strategies
Effective normalization is crucial for accurate quantification:
- Use exogenous spike-ins (e.g., cel-miR-39-3p, cel-miR-54-3p) added during lysis or RNA extraction to control for technical variability [51] [18]
- Prepare serial dilutions of spike-ins (0.001-1000 ng/μL) for validation and standardization [18]
- For duplex assays, use ratio-based normalization (e.g., miR-4488/miR-579-3p ratio) which may provide superior analytical performance [53]
Applications and Validation Studies
Clinical Validation in Disease Biomarker Discovery
dPCR has demonstrated exceptional performance in multiple clinical research applications:
- Cardiovascular Disease: Extraction-free dPCR successfully quantified miR-133a in acute myocardial infarction patients versus healthy controls, establishing its clinical utility [51]
- Metastatic Melanoma: Duplex dPCR assay simultaneously quantified miR-4488 and miR-579-3p in serum, with the expression ratio (miRatio) showing strong prognostic value for MAPK inhibitor response [53]
- Hepatocellular Carcinoma: An optimized ddPCR assay with LNA-modified probes precisely quantified miR-192-5p in patient plasma, demonstrating significantly reduced levels in cancer patients (444.2 vs. 753.5 copies/μL, p<0.001) [55]
- Medication-Related Osteonecrosis of the Jaw (MRONJ): dPCR identified differentially expressed miRNAs (miR-483-5p, miR-92-5p, miR-628-3p, and miR-486-5p) in patient plasma and exosomes [3]
Multiplex dPCR Assay Development
Recent advances enable simultaneous quantification of multiple miRNAs:
- Design target-specific primers and differentially labeled probes (FAM, VIC, etc.) [53]
- Optimize primer concentrations and thermal cycling conditions to maintain efficiency across targets [53] [56]
- Validate using singleplex reactions as reference [53]
- Apply to ratio-based biomarkers (e.g., oncogenic/tumor suppressor miRNA ratios) for improved clinical accuracy [53]
Table 2: Representative Clinical Applications of dPCR for miRNA Quantification
| Disease Area | Key miRNA Targets | Sample Type | Performance Characteristics |
|---|---|---|---|
| Acute Myocardial Infarction | miR-133a | Plasma | Successful differentiation of patients vs. controls using extraction-free protocol [51] |
| Metastatic Melanoma | miR-4488, miR-579-3p (ratio) | Serum | Predictive of MAPKi response; duplex assay format [53] |
| Hepatocellular Carcinoma | miR-192-5p | Plasma | AUC=0.70; improved to AUC=0.88 in multi-marker model [55] |
| MRONJ | miR-483-5p, miR-92-5p, miR-628-3p, miR-486-5p | Plasma and exosomes | Consistent differential expression in both plasma and exosomal fractions [3] |
| Lung Cancer | miR-21, miR-126, let-7a | Serum | Superior precision vs. qPCR, particularly for let-7a [52] |
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents and Materials for dPCR miRNA Quantification
| Reagent/Equipment | Function/Purpose | Example Products/Suppliers |
|---|---|---|
| RNA Isolation Kits | Plasma miRNA extraction | miRNeasy Serum/Plasma Kit (Qiagen), Total RNA Purification Plus Kit (Norgen) [53] [20] |
| cDNA Synthesis Kits | Reverse transcription of miRNAs | TaqMan Advanced miRNA cDNA Synthesis Kit (Thermo Fisher) [51] [3] |
| dPCR Systems | Partitioning, amplification, and reading | QuantStudio 3D Digital PCR System (Thermo Fisher), QIAcuity (Qiagen), Bio-Rad ddPCR System [51] [54] |
| TaqMan miRNA Assays | Target-specific detection | TaqMan MicroRNA Assays (Thermo Fisher) with FAM/VIC labels [51] [53] |
| Exogenous Controls | Normalization and process control | cel-miR-39-3p, cel-miR-54-3p (synthetic miRNAs not found in humans) [51] [18] |
| dPCR Consumables | Reaction partitioning | QuantStudio 3D chips, DG8 Cartridges (Bio-Rad), QIAcuity nanoplate [51] [18] [54] |
Workflow Visualization: dPCR for Plasma miRNA Analysis
Diagram 1: Comprehensive workflow for plasma miRNA quantification using digital PCR, highlighting both conventional and extraction-free protocols.
Digital PCR represents a significant advancement in plasma miRNA quantification, addressing critical limitations of traditional RT-qPCR methodologies. The capacity for absolute quantification without standard curves, superior precision for low-abundance targets, and compatibility with simplified workflows including extraction-free protocols position dPCR as an indispensable tool for biomarker discovery and validation. As the field moves toward multiplexed assays and ratio-based biomarkers, dPCR platforms offer the technical robustness required for clinical translation of miRNA signatures across diverse disease areas.
Researchers implementing dPCR for plasma miRNA quantification should prioritize appropriate normalization strategies, rigorous assay optimization, and validation in clinically relevant sample sets to fully leverage the capabilities of this powerful technology.
Solving Common Problems: Quality Control, Inhibition, and Data Normalization
Accurate measurement of circulating microRNAs (miRNAs) in plasma is a critical component of modern molecular diagnostics and biomarker research [57]. These molecules are promising biomarkers for a range of ageing-related diseases, including Alzheimer's disease and various cancers, due to their stability in biofluids and disease-specific expression patterns [13]. However, the reliability of miRNA quantification using RT-qPCR is significantly compromised by hemolysis, the rupture of red blood cells (RBCs) during blood collection or sample processing, which releases intracellular miRNAs and contaminates the plasma miRNA profile [58] [59].
The miRNA content released from blood cells during hemolysis can dramatically alter the expression of certain miRNAs, potentially leading to the false discovery of disease-associated biomarkers [58]. In fact, previous research has indicated that over half of the proposed miRNA biomarkers for solid cancers are highly expressed in one or more blood cell types, making them particularly vulnerable to hemolysis interference [58]. Consequently, accurate detection and quantification of hemolysis has become an essential quality control step in plasma miRNA research.
Two principal methods have emerged as gold standards for hemolysis assessment in plasma samples: spectrophotometric measurement of hemoglobin absorbance and quantitative assessment of miRNA ratios, particularly the ratio of miR-451a to miR-23a-3p [13] [59]. This application note provides a detailed comparison of these methodologies and offers standardized protocols for their implementation within RT-qPCR workflows for plasma miRNA quantification.
Comparative Analysis of Hemolysis Detection Methods
Principle of Detection
Spectrophotometric Absorbance Method: This technique leverages the optical properties of hemoglobin, the predominant protein released during RBC lysis. Free hemoglobin in plasma has a characteristic absorbance spectrum with a peak at 414 nm (A₄₁₄) [59] [60]. The degree of absorbance at this wavelength is directly proportional to the concentration of cell-free hemoglobin in the sample, which in turn correlates with the extent of hemolysis [58].
miRNA Ratio Method (miR-451a/miR-23a-3p): This approach uses reverse transcription quantitative PCR (RT-qPCR) to measure the ratio between a RBC-enriched miRNA and a stable reference miRNA in plasma. miR-451a is highly abundant in RBCs, and its levels increase significantly in plasma upon hemolysis. In contrast, miR-23a-3p remains relatively stable and unaffected by hemolysis, serving as an invariant control [58] [13]. The difference between their quantification cycles (ΔCq = Cq miR-23a-3p - Cq miR-451a) provides a sensitive indicator of RBC contamination.
Performance Comparison
Direct comparison studies have revealed significant differences in sensitivity and performance between these hemolysis detection methods.
Table 1: Comparison of Sensitivity and Performance Characteristics
| Method | Principle | Detection Limit | Key Advantages | Key Limitations |
|---|---|---|---|---|
| miRNA Ratio (miR-451a/miR-23a-3p) | ΔCq measurement via RT-qPCR | ~0.001% hemolysis [58] | High sensitivity, integrates with miRNA workflow, specific to RBC miRNA contamination | Requires RNA extraction and RT-qPCR, more time-consuming, higher cost |
| Spectrophotometry (A₄₁₄) | Hemoglobin absorbance at 414 nm | ~0.004% hemolysis [58] | Rapid, cost-effective, requires minimal sample volume | Less sensitive than miRNA ratio, potential interference from other pigments |
| Visual Inspection | Pink discoloration of plasma | ~1-5% hemolysis (estimated) [58] | Simple, no equipment needed | Subjective, very low sensitivity, unreliable |
| Automated Analyzer | Hemoglobin concentration measurement | ~1% hemolysis [58] | Quantitative, automated | Low sensitivity, requires specialized clinical analyzer |
The data clearly demonstrates the superior sensitivity of the miRNA ratio method, capable of detecting hemolysis at levels approximately 250 times lower than visual inspection and significantly lower than spectrophotometry [58]. This is critical because miRNA profiles can be altered by hemolysis before it becomes visually detectable [58].
Table 2: Interpretation Guidelines for Hemolysis Assays
| Method | Low/No Hemolysis | Moderate Hemolysis | High Hemolysis |
|---|---|---|---|
| ΔCq (miR-23a-3p - miR-451a) | > 7 [13] [59] | 5 - 7 [58] | < 5 [58] |
| A₄₁₄ Absorbance Cutoff | < 0.072 [58] | 0.072 - 0.3 [58] | > 0.3 [58] |
Detailed Experimental Protocols
Protocol 1: Spectrophotometric Assessment of Hemolysis
Principle: Direct measurement of free hemoglobin in plasma or serum by quantifying absorbance at 414 nm (A₄₁₄) using a spectrophotometer or plate reader.
Materials and Reagents
Table 3: Research Reagent Solutions for Spectrophotometric Hemolysis Detection
| Item | Function/Description | Example |
|---|---|---|
| Spectrophotometer/Plate Reader | Measures absorbance at specific wavelengths (414 nm) | NanoDrop 1000 [58] |
| Cuvettes/Microplates | Holds sample for absorbance measurement | Clear UV-transparent plates |
| Pipettes and Tips | Accurate liquid handling | - |
| Plasma/Serum Sample | Cell-free blood fraction to be tested | - |
Procedure
- Sample Preparation: Centrifuge blood samples collected in EDTA or heparin tubes to obtain clear plasma (e.g., 800 × g for 15 minutes at 4°C, followed by a second centrifugation if needed) [59] [60]. For serum, allow blood to clot in serum tubes for 15-30 minutes before centrifugation [58].
- Blank Instrument: Use the corresponding buffer (e.g., PBS) or elution buffer as a blank.
- Measure Absorbance: Pipette 2-3 µL of clear plasma or serum onto the spectrophotometer pedestal or into a cuvette. Record the absorbance at 414 nm (A₄₁₄). For higher throughput, use a microplate reader with appropriately diluted samples in a plate format.
- Data Interpretation: Classify samples based on established cut-offs. Samples with an A₄₁₄ < 0.072 are generally considered acceptable for miRNA analysis, while those with A₄₁₄ > 0.3 indicate significant hemolysis [58].
Protocol 2: miRNA Ratio Assessment of Hemolysis via RT-qPCR
Principle: Quantification of RBC-specific miR-451a and stable miR-23a-3p to calculate a ΔCq value as a sensitive indicator of hemolysis.
Materials and Reagents
Table 4: Research Reagent Solutions for miRNA Ratio Hemolysis Detection
| Item | Function/Description | Example |
|---|---|---|
| RNA Extraction Kit | Isolation of total RNA, including miRNAs, from plasma | miRCURY RNA Isolation Kit for Biofluids [58] |
| Carrier RNA | Enhances RNA precipitation and recovery from low-concentration samples | MS2 bacteriophage carrier RNA [58] |
| Reverse Transcription Kit | Converts miRNA to cDNA using specific stem-loop primers | Universal cDNA Synthesis Kit [58] |
| qPCR Master Mix | Contains enzymes, dNTPs, buffer, and fluorescent dye for PCR | ExiLENT SYBR Green master mix [58] |
| miRNA-specific PCR Assays | LNA-enhanced primers for miR-451a and miR-23a-3p | Pick-&-Mix microRNA PCR panels [58] |
| Real-time PCR System | Instrument for performing and quantifying PCR amplification | 7900HT Fast Real-Time PCR System [58] |
Procedure
RNA Extraction:
Reverse Transcription (RT):
- Perform RT reactions using stem-loop primers specific to miR-451a and miR-23a-3p. This structure improves specificity and sensitivity for short miRNA templates [45].
- Use 2-5 µL of extracted RNA in a 10-20 µL RT reaction [58].
- Include multiple RT replicates (e.g., 3 independent reactions per sample) to account for technical variability [58].
Quantitative PCR (qPCR):
- Prepare a PCR master mix containing diluted cDNA, SYBR Green dye, and miRNA-specific forward and universal reverse primers.
- Use LNA (Locked Nucleic Acid)-enhanced primers for increased specificity and binding affinity [58].
- Perform amplification on a real-time PCR system with the following typical cycling conditions: 95°C for 10 minutes, followed by 40 cycles of 95°C for 10 seconds and 60°C for 1 minute [58].
Data Analysis:
- Record the quantification cycle (Cq) for miR-451a and miR-23a-3p.
- Calculate the ΔCq value using the formula: ΔCq = Cq (miR-23a-3p) - Cq (miR-451a).
- Interpret the results: A ΔCq < 5 suggests a high risk of hemolysis, a ΔCq between 5-7 suggests moderate risk, and a ΔCq > 7 suggests the sample is clear of significant RBC contamination [58] [13].
Integrated Workflow and Recommendations for miRNA Studies
The following workflow diagram illustrates the recommended procedure for implementing hemolysis checks in a plasma miRNA study, combining the speed of absorbance screening with the sensitivity of the miRNA ratio for critical samples.
Diagram Title: Hemolysis Assessment Workflow for Plasma miRNA Studies
For reliable miRNA analysis in plasma, the following integrated approach is recommended:
- Initial Rapid Screening: Employ the spectrophotometric A₄₁₄ method for all incoming samples due to its speed and low cost. This quickly identifies grossly hemolyzed samples [58].
- Confirmatory Testing: For samples that show moderate A₄₁₄ absorbance or are intended for critical biomarker discovery studies, proceed with the more sensitive miRNA ratio method. This step is essential as it specifically detects the miRNA contamination that directly impacts RT-qPCR results [13] [59].
- Standardized Reporting: Always report the method and metrics used for hemolysis assessment (A₄₁₄ value and/or ΔCq ratio) in publications to improve reproducibility and allow for cross-study comparisons [13].
- Assay Consistency: For RT-qPCR analysis, perform all steps, including normalization, using the same machine and software throughout the entire study to minimize technical variability [13].
Both spectrophotometric absorbance and the miRNA ratio (miR-23a-3p/miR-451a) are valid methods for hemolysis detection, yet they serve complementary roles. The absorbance method offers a rapid, cost-effective initial screen, while the miRNA ratio provides a highly sensitive, miRNA-specific confirmation. Integrating both methods into a standardized workflow, as detailed in this application note, is crucial for ensuring the reliability and accuracy of plasma miRNA quantification in RT-qPCR-based research and diagnostic development. Robust hemolysis assessment is a non-negotiable pre-analytical step for generating high-quality, reproducible data in circulating miRNA studies.
Identifying and Minimizing PCR Inhibitors from Sample Matrix and Residual Reagents
The accurate quantification of circulating microRNAs (miRNAs) in plasma using reverse transcription quantitative polymerase chain reaction (RT-qPCR) is a cornerstone of modern biomarker research. These short, noncoding RNA molecules offer immense promise as minimally invasive biomarkers for diagnosing, prognosticating, and monitoring a wide array of human diseases, including ageing-related conditions like Alzheimer's [13]. However, the translational potential of these biomarkers is often hampered by a lack of reproducibility between studies. A significant contributor to this inconsistency is the presence of PCR inhibitors derived from the original sample matrix or introduced during the RNA isolation process [61]. These inhibitors can lead to suppressed amplification, resulting in underestimation of miRNA levels and potentially false conclusions.
The challenge is particularly acute in plasma samples, where the concentration of target miRNAs is exceptionally low. This low abundance makes the RT-qPCR reaction highly susceptible to even trace amounts of interfering substances, which can co-purify with the RNA [61]. This application note, framed within the context of developing a robust RT-qPCR protocol for plasma miRNA research, provides a detailed guide to identifying the primary sources of PCR inhibitors and implementing effective strategies to minimize their impact, thereby ensuring the reliability of experimental data.
Understanding the origin and nature of PCR inhibitors is the first step toward mitigating their effects. These inhibitors can be broadly categorized into two groups: those inherent to the sample matrix and those introduced as residual reagents during nucleic acid extraction.
Sample Matrix-Derived Inhibitors
The plasma matrix itself is a complex mixture of components that can interfere with the enzymatic reactions of RT-qPCR.
- Hemolysis: This is a major pre-analytical challenge. The lysis of red blood cells releases intracellular miRNAs, such as the erythrocyte-enriched miR-451a, which can dramatically alter the perceived miRNA profile and mask disease-specific signals [62] [13]. Hemoglobin and other cellular components released during hemolysis are also potent PCR inhibitors.
- Plasma Components: Endogenous biomolecules such as proteins (e.g., immunoglobulins), lipids, carbohydrates, and bile salts can inhibit polymerase activity. Their concentrations can vary with dietary status, the type of anticoagulant used (e.g., EDTA, heparin), and sample storage conditions [61].
- Complex Organic Compounds: In environmental water testing, which shares analytical challenges with complex clinical samples, humic acids, polyphenols, and metal ions (e.g., calcium) are well-documented inhibitors. Calcium ions, for instance, can compete with the magnesium co-factors essential for DNA polymerase activity [63].
Residual Reagent-Derived Inhibitors
The process of RNA extraction, while necessary for purification, can leave behind reagents that inhibit downstream applications.
- Phenol: Chloroform-phenol-based extraction methods, while effective, are prone to leaving residual phenol in the final RNA eluate. Residual phenol can interfere with both the spectrophotometric quantification of RNA and the enzymatic efficiency of RT-qPCR [61].
- Chaotropic Salts: Guanidinium thiocyanate and guanidine hydrochloride are powerful denaturants used in lysis buffers to inactivate nucleases. While excellent for preserving RNA integrity, they are potent PCR inhibitors. Inadequate washing during solid-phase extraction can leave these salts behind [64] [63].
- Alcohols: Ethanol and isopropanol, used in washing steps, can inhibit PCR if not completely removed [61].
Table 1: Common PCR Inhibitors and Their Sources
| Inhibitor Category | Specific Examples | Primary Source | Mechanism of Interference |
|---|---|---|---|
| Sample Matrix | Hemoglobin, Lactoferrin | Hemolyzed plasma/serum | Binds to polymerase; degrades nucleic acids |
| Immunoglobulins, Albumin | Plasma | Binds to single-stranded DNA | |
| Lipids, Polysaccharides | Plasma | Interferes with cell lysis & nucleic acid binding | |
| Heparin | Plasma (Anticoagulant) | Competes with polymerase for magnesium ions | |
| Residual Reagents | Guanidinium salts | Lysis/binding buffer | Denatures enzymes in RT/qPCR |
| Phenol | Phenol-chloroform extraction | Denatures enzymes; affects UV quantification | |
| Ethanol/Isopropanol | Wash buffers | Disrupts enzymatic reaction kinetics | |
| Environmental | Humic & Fulvic Acids | Complex water samples | Binds to nucleic acids and polymerase |
Technical Strategies for Inhibitor Identification and Minimization
A multi-faceted approach involving rigorous quality control and optimized protocols is essential for managing PCR inhibition.
Quality Control: Spike-in Controls and Hemolysis Assessment
The use of exogenous spike-in controls is a powerful strategy to monitor technical performance across the entire workflow.
- Isolation Spike-ins: A mix of synthetic RNA molecules (e.g., based on C. elegans miRNAs like cel-miR-54) should be added to the plasma sample prior to RNA isolation. These controls assess the efficiency of the RNA extraction process. Significant deviations in their quantification cycle (Cq) values indicate poor or variable recovery [62].
- RT Spike-ins: A second set of spike-ins (e.g., cel-miR-76) should be added prior to the reverse transcription reaction. These controls monitor the efficiency of the cDNA synthesis and the presence of inhibitors in the final RNA eluate that could affect the RT and PCR enzymes. The difference in Cq (ΔCq) between high-abundance and low-abundance RT spike-ins should fall within an expected range (e.g., 5.5–6.5 cycles); a larger ΔCq suggests possible inhibition [62].
Hemolysis must be quantitatively assessed. An effective method is to measure the ratio of miR-23a-3p (a miRNA stable in plasma) to miR-451a (a miRNA highly abundant in erythrocytes) via RT-qPCR. A ΔCq (miR-23a-3p – miR-451a) of less than 7 is generally considered acceptable, with lower values indicating significant hemolysis [13]. This method is particularly useful when the original sample is no longer available for visual or spectrophotometric inspection.
Optimized Nucleic Acid Extraction
The choice of extraction method critically influences the purity and yield of RNA, directly impacting downstream PCR efficiency.
- Magnetic Bead-Based Methods: Recent studies demonstrate that magnetic bead-based extraction methods often outperform traditional column-based kits for challenging samples. For instance, in the extraction of bacterial DNA from whole blood—a matrix with similar inhibitory challenges to plasma—a magnetic bead-based system (GraBon) showed a 77.5% accuracy for S. aureus detection, compared to 67.5% for a column-based method (QIAamp DNA Blood Mini Kit) [65]. The key advantage is the ability to include a step that isolates bacteria from inhibitory blood components before lysis.
- Protocol Optimizations: The fundamental Boom method using silica beads can be significantly optimized. Key parameters include:
- Binding pH: Using a binding buffer at a lower pH (e.g., pH 4.1 vs. 8.6) reduces the negative charge on silica, enhancing nucleic acid binding efficiency. One study showed 98.2% of input DNA bound at pH 4.1 versus 84.3% at pH 8.6 [64].
- Efficient Mixing: A "tip-based" mixing method, where the binding mix is aspirated and dispensed repeatedly, was shown to achieve ~85% DNA binding within 1 minute, compared to only ~61% with standard orbital shaking [64].
- Carriers: Adding carriers like glycogen during RNA isolation can significantly improve yield and reproducibility by reducing adsorptive losses, without negatively impacting RT-qPCR [62].
Table 2: Comparison of Nucleic Acid Extraction Method Performance
| Extraction Method | Example Product | Key Features | Reported Performance | Advantages/Limitations |
|---|---|---|---|---|
| Magnetic Bead-Based | GraBon system | Automated; bacterial isolation pre-lysis | 77.5% accuracy for S. aureus in WB [65] | Adv: High purity, automatable, high throughputLim: Higher initial instrument cost |
| Magnetic Bead-Based | K-SL DNA Extraction Kit | Manual; bacterial isolation pre-lysis | 77.5% accuracy for E. coli in WB [65] | Adv: High purity, effective inhibitor removalLim: Manual processing |
| Silica Column-Based | QIAamp DNA Blood Mini Kit | Direct lysis in whole blood | 65.0% accuracy for E. coli in WB [65] | Adv: Widely used, benchmarkedLim: Co-processing of inhibitors, lower sensitivity |
| SHIFT-SP | (Research method) | Optimized magnetic bead (fast, tip-based mixing) | ~85% DNA binding in 1 min; high-yield [64] | Adv: Speed (6-7 min), high yield, automation-compatibleLim: Not yet commercial |
Inhibitor Removal and PCR Amplification Enhancements
If inhibition is detected in the purified nucleic acids, several strategies can be employed post-extraction.
- Sample Dilution: Diluting the RNA template is a simple and effective way to reduce the concentration of inhibitors to a level below their inhibitory threshold. The optimal dilution factor must be determined empirically, as over-dilution can cause the target to fall below the limit of detection [63].
- Polymeric Adsorbents: Treating samples with polymers like Supelite DAX-8 can permanently remove humic acids and other organic inhibitors. One study found that 5% DAX-8 treatment increased the detectable levels of murine norovirus (a process control) by RT-qPCR in complex environmental water samples, outperforming other inhibitor removal kits [63].
- PCR Additives: Adding certain compounds to the PCR master mix can help counteract inhibitors. Bovine Serum Albumin (BSA) acts as a "competitor" for binding inhibitors, freeing up the polymerase. Dithiothreitol (DTT) can help break down inhibitors like polyphenols. T4 gene 32 protein is also known to enhance amplification in the presence of inhibitors [63].
Experimental Protocols
Protocol: Assessment of Hemolysis and Technical Performance using RT-qPCR
Purpose: To evaluate plasma sample quality for miRNA analysis by assessing hemolysis and quantifying the efficiency of RNA isolation and reverse transcription.
Materials:
- Isolated RNA from plasma samples
- Two-tailed RT-qPCR assays for miR-23a-3p, miR-451a [62] [13]
- Synthetic spike-in RNA mixes (Isolation spike-ins: e.g., cel-miR-54, spike-A, spike-B; RT spike-ins: e.g., cel-miR-76, cel-miR-2) [62]
- RT-qPCR reagents and instrumentation
Procedure:
- Spike-in Addition:
- During RNA Isolation: Add a known, constant amount of the isolation spike-in mix (cel-miR-54, spike-A, spike-B) to each plasma sample before RNA extraction.
- During Reverse Transcription: Add a known amount of the RT spike-in mix (cel-miR-76, cel-miR-2) to the RNA eluate before the RT reaction.
- RT-qPCR Analysis: Perform reverse transcription and qPCR for the following targets in each sample:
- Endogenous miRNAs: miR-23a-3p and miR-451a
- Isolation spike-ins: cel-miR-54, spike-A, spike-B
- RT spike-ins: cel-miR-76, cel-miR-2
- Data Interpretation:
- Hemolysis Assessment: Calculate ΔCqhemolysis = Cq(miR-23a-3p) – Cq(miR-451a).
- ΔCqhemolysis < 5: Indicates significant hemolysis; consider excluding the sample.
- ΔCq_hemolysis ~7: Acceptable, minimal hemolysis [13].
- Isolation Efficiency: The ΔCq's between the high, moderate, and low abundance isolation spike-ins should be consistent and within an expected range (e.g., 3.5–5.5 cycles). Large deviations indicate poor or variable RNA recovery.
- Inhibition Monitoring: The ΔCq between the high and low abundance RT spike-ins (Cq(cel-miR-76) – Cq(cel-miR-2)) should be stable (e.g., 5.5–6.5 cycles). A larger ΔCq suggests the presence of inhibitors in the RNA eluate [62].
- Hemolysis Assessment: Calculate ΔCqhemolysis = Cq(miR-23a-3p) – Cq(miR-451a).
Protocol: High-Yield, Rapid RNA Extraction using Optimized Magnetic Bead Principles
Purpose: To extract high-purity RNA from plasma with minimal co-purification of inhibitors, based on the optimized SHIFT-SP method [64].
Materials:
- Plasma samples
- Lysis/Binding Buffer (LBB) with guanidinium thiocyanate, pH adjusted to ~4.1
- Magnetic silica beads
- Wash buffers (e.g., ethanol-based)
- Nuclease-free water or low-ionic-strength elution buffer
- A thermal shaker or a pipettor capable of vigorous tip-based mixing
Procedure:
- Lysis: Mix plasma sample with Lysis/Binding Buffer thoroughly.
- Binding: Add magnetic silica beads to the lysate.
- For manual processing, use a "tip-based" mixing method: Aspirate and dispense the entire binding mixture (beads + lysate) vigorously for 1-2 minutes using a pipette [64].
- Alternatively, incubate with vigorous orbital shaking for 10-15 minutes at room temperature.
- Bead Capture: Place the tube on a magnetic stand until the solution clears. Carefully remove and discard the supernatant.
- Washing: Wash the beads twice with an ethanol-based wash buffer. Capture the beads on the magnet between each wash and remove the supernatant completely.
- Drying: Briefly air-dry the bead pellet to evaporate residual ethanol.
- Elution: Elute the RNA in nuclease-free water or a specified elution buffer by incubating at an elevated temperature (e.g., 65-80°C) for 2-5 minutes. Capture the beads and transfer the eluate containing purified RNA to a new tube.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Reagents for Managing PCR Inhibition
| Reagent / Kit | Function | Application Note |
|---|---|---|
| Synthetic RNA Spike-ins (e.g., cel-miR-39, cel-miR-54, cel-miR-76) | Exogenous controls for quality control | Added to sample pre-isolation and/or pre-RT to monitor RNA yield, reverse transcription efficiency, and PCR inhibition [62]. |
| miRNeasy Serum/Plasma Advanced Kit (Qiagen) | Column-based RNA isolation | Optimized for low-abundance miRNA from biofluids. Performance can be improved by adding glycogen as a carrier [62]. |
| Magnetic Silica Beads | Solid-phase nucleic acid binding | Core of many modern, automatable extraction kits. Binding efficiency is highly dependent on buffer pH and mixing mode [64]. |
| Supelite DAX-8 | Polymeric adsorbent | Added to samples post-concentration/pre-extraction to remove humic acids and other organic PCR inhibitors [63]. |
| Bovine Serum Albumin (BSA) | PCR additive | Added to the PCR master mix to bind and neutralize a wide range of inhibitors, improving amplification efficiency [63]. |
Workflow and Strategic Decision Diagram
The following diagram outlines a logical workflow for identifying and mitigating PCR inhibitors in a plasma miRNA study, from sample collection to data interpretation.
Workflow for Managing PCR Inhibitors in Plasma miRNA Analysis
This workflow provides a structured, quality-controlled pathway for plasma miRNA analysis. It begins with sample collection and an initial check for hemolysis—a major source of bias and inhibition. Samples failing this check should be excluded. Successful samples undergo RNA extraction with isolation spike-in controls to monitor yield. If isolation is inefficient, the protocol itself should be re-optimized. Following reverse transcription with RT spike-in controls, a final QC step checks for inhibitors in the eluate. If inhibition is detected, post-extraction mitigation strategies like dilution or additive use can be applied before proceeding with final, reliable miRNA quantification.
Within the framework of developing a robust RT-qPCR protocol for microRNA (miRNA) quantification in plasma, optimizing amplification efficiency is a critical step for generating reliable, publication-quality data. Circulating miRNAs in plasma and serum are promising biomarkers for various human diseases, including cancer, neurological, and metabolic disorders [20]. However, their low abundance and the complex composition of plasma present significant challenges for quantitative reverse transcription PCR (RT-qPCR). This application note provides detailed methodologies and structured data to guide researchers in fine-tuning annealing temperatures and reaction conditions to achieve optimal amplification efficiency, a prerequisite for accurate relative quantification using methods like the 2−ΔΔCt approach [66].
The Critical Role of Amplification Efficiency
In qPCR, amplification efficiency (E) refers to the fraction of target templates that duplicates in each PCR cycle. An efficiency of 100% (E=2) represents a perfect doubling. Efficiencies between 90% and 110% are generally considered acceptable for reliable relative quantification [67]. Several factors can cause efficiency to deviate from this ideal range:
- Efficiency >100%: This is often an artifact caused by the presence of polymerase inhibitors in concentrated samples. These inhibitors, which can include carryover contaminants from the RNA isolation process, are diluted out in subsequent sample dilutions, leading to a miscalculation of the slope and an overestimation of efficiency [67].
- Efficiency <90%: This is typically caused by non-optimal reaction conditions, such as suboptimal annealing temperature, poor primer design, or inadequate reagent concentrations [67].
For miRNA quantification from plasma, these challenges are compounded by low starting RNA concentrations and potential inhibitors co-purified during RNA extraction [68]. Therefore, a systematic optimization of the protocol is not just beneficial but essential.
Key Parameters for Optimization
The following parameters are fundamental levers for optimizing the efficiency and specificity of your RT-qPCR assay for plasma miRNAs.
Annealing Temperature
The annealing temperature is perhaps the most crucial parameter to optimize. Using a temperature that is too low can lead to non-specific amplification and primer-dimer formation, while a temperature that is too high can reduce yield or prevent amplification entirely.
- Initial Testing: It is recommended to perform a temperature gradient RT-qPCR experiment, testing a range around the calculated primer Tm [69]. For miRNA assays using stem-loop primers, the manufacturer's recommended temperature is a good starting point.
- Optimal Result: The optimal annealing temperature produces the lowest Cq value with the highest fluorescence (ΔRn) and a single, sharp peak in the melt curve analysis, indicating a single specific amplification product.
Primer and Probe Design
While pre-designed assay kits (e.g., TaqMan assays) circumvent the need for manual design [70], custom designs require careful attention.
- Amplicon Length: Short PCR amplicons, ranging from 70 to 200 bp, are recommended for maximum PCR efficiency [69]. This is naturally achieved for mature miRNAs.
- GC Content: Target sequences should ideally have a GC content of 40–60% [69].
- Specificity: For custom mRNA assays, primers should be designed to span an exon-exon junction to prevent amplification of genomic DNA [48].
Reaction Composition and Template Quality
- Template Input: The optimal amount of input RNA must be determined empirically. For plasma samples, where RNA concentration is low, a pre-amplification step is often incorporated [70]. Testing a dilution series of your cDNA is crucial to identify the linear range and avoid inhibition [67].
- Primer and Probe Concentrations: Optimal concentrations for dye-based and probe-based experiments are typically 400 nM and 200 nM, respectively, but may be optimized between 100–900 nM and 100–500 nM [69].
- Plasma-Specific Considerations: The use of silica column–based RNA extraction methods is more effective and reliable for plasma samples compared to TRIzol-based methods [17]. Furthermore, the stability of plasma miRNAs must be considered; samples should be stored at -70°C to prevent miRNA degradation [17].
Table 1: Optimization Parameters and Their Recommended Ranges
| Parameter | Recommended Range | Considerations for Plasma miRNA |
|---|---|---|
| Annealing Temperature | Gradient testing ± 5°C from Tm [69] | Pre-designed assay recommendations are a valid starting point [70]. |
| Amplicon Length | 70–200 bp [69] | Mature miRNAs are short; amplicons will inherently be small. |
| Primer Concentration | 100–900 nM (Optimal: 400 nM) [69] | High concentrations can increase spurious amplification. |
| Probe Concentration | 100–500 nM (Optimal: 200 nM) [69] | Ensure the probe Tm is 5–10°C higher than the primer Tm [69]. |
| Template Input | Dilution series to find linear range [67] | Low concentration in plasma; pre-amplification may be necessary [68] [70]. |
| PCR Efficiency (E) | 90% – 110% [67] | Calculate from a standard curve of serial dilutions. |
Experimental Protocol: A Stepwise Optimization Workflow
The following protocol provides a detailed step-by-step guide for systematically optimizing RT-qPCR conditions, with a focus on annealing temperature and reaction composition.
Step 1: RNA Isolation and Quality Control
- Isolation: Extract total RNA from plasma using a dedicated kit, such as the miRNeasy Serum/Plasma Kit (Qiagen) or the MagMAX mirVana Total Isolation Kit (Thermo Fisher) [68]. Silica column-based methods are preferred [17].
- Spike-in Controls: Incorporate exogenous spike-in controls (e.g., cel-miR-39) during the lysis step to monitor isolation efficiency and normalize for technical variability [17] [13].
- Haemolysis Check: Assess sample haemolysis using an absorbance-based method (A414/A380) or a miRNA ratio method (ΔCq of miR-23a-3p and miR-451a) to exclude haemolysed samples that can skew miRNA profiles [13].
Step 2: Reverse Transcription
- Choice of Chemistry: Use a kit specifically designed for miRNA, such as the TaqMan MicroRNA Reverse Transcription Kit (best for 1-10 targets) or the TaqMan Advanced miRNA cDNA Synthesis Kit (for a streamlined workflow with universal RT) [70].
- Priming Strategy: For two-step assays, a mixture of random primers and oligo(dT) primers can help improve the reverse transcription efficiency and qPCR sensitivity [48].
Step 3: Optimization of qPCR Conditions
This is the core optimization procedure.
- Prepare a Master Mix for your chosen number of reactions, including all components except the template. Use a master mix suitable for your detection method (e.g., intercalating dye or probe-based).
- Design the Experiment: For a comprehensive optimization, test a matrix of different annealing temperatures (e.g., a gradient from 55°C to 65°C) and primer concentrations (e.g., 200 nM, 400 nM, 600 nM).
- Set Up Reactions: Dispense the master mix into the wells of a qPCR plate. Include a no-template control (NTC) for each condition to check for contamination and a no-reverse-transcription control (-RT) to assess genomic DNA contamination [48] [69].
- Add Template: Use a standardized, diluted cDNA sample as the template across all test reactions.
- Run qPCR Program: Use the following cycling conditions as a base, modifying the annealing temperature according to the gradient being tested:
- Initial Denaturation: 95°C for 2 minutes (if using a Hot Start polymerase).
- Amplification (40-45 cycles):
- Denature: 95°C for 15 seconds.
- Anneal/Extend: [Temperature Gradient] for 30-60 seconds. (Data collection occurs in this step)
- Melt Curve Analysis: 60°C to 95°C, with a gradual increase (0.3°C/sec) if using an intercalating dye.
Step 4: Data Analysis and Efficiency Calculation
- Analyze Amplification Curves: Identify the conditions that yield the lowest Cq value and highest ΔRn.
- Check Melt Curves: Confirm that the optimal condition produces a single, sharp peak.
- Calculate PCR Efficiency:
- Prepare a standard curve using a serial dilution (e.g., 1:10, 1:100, 1:1000) of a pooled cDNA sample.
- Run qPCR on these dilutions under the optimized conditions.
- Plot the Log10(Starting Quantity) against the Cq value for each dilution.
- Perform linear regression to obtain the slope of the trendline.
- Calculate efficiency using the formula: E = -1 + 10(-1/slope).
- The optimal condition should yield a linear dynamic range with a correlation coefficient (R²) ≥ 0.99 and an efficiency between 90% and 110% [66] [67].
The following workflow diagram illustrates the key steps in this optimization process:
Figure 1: RT-qPCR Optimization Workflow. This diagram outlines the sequential steps for systematically optimizing RT-qPCR conditions for plasma miRNA quantification.
The Scientist's Toolkit: Research Reagent Solutions
Success in miRNA quantification relies on using appropriate, validated reagents and controls. The following table details key materials and their functions.
Table 2: Essential Reagents and Kits for Plasma miRNA RT-qPCR
| Item | Function | Example Products |
|---|---|---|
| miRNA Isolation Kit | Efficiently extracts small RNAs from plasma/serum; column-based methods are preferred. | miRNeasy Serum/Plasma Kit (Qiagen) [20] [68], MagMAX mirVana Total Isolation Kit (Thermo Fisher) [68], TaqMan miRNA ABC Purification Kit (Thermo Fisher) [70] |
| Spike-in Synthetic miRNA | Exogenous control for normalizing extraction and RT efficiency. | cel-miR-39 [17], UniSp6 [68] |
| Reverse Transcription Kit | Converts miRNA to cDNA; specific chemistries exist for miRNA. | TaqMan MicroRNA RT Kit (for 1-10 targets) [70], TaqMan Advanced miRNA cDNA Synthesis Kit (universal RT) [70] |
| Pre-Amplification Kit | Increases cDNA quantity for low-input samples like plasma. | Megaplex PreAmp Primers [70] |
| qPCR Master Mix | Contains polymerase, dNTPs, and buffer for detection. | TaqMan Fast Advanced Master Mix [70], Luna Universal Probe One-Step RT-qPCR Kit (NEB) [69] |
| Validated miRNA Assays | Pre-designed primers and probes for specific, sensitive detection. | TaqMan MicroRNA Assays [20] [70] |
| Stable Normalizer miRNAs | Endogenous controls for data normalization. | miR-16-5p, miR-223-3p [20] [13] |
A meticulously optimized RT-qPCR protocol is the foundation for accurate and reproducible quantification of circulating miRNAs in plasma. By systematically evaluating and adjusting key parameters—especially annealing temperature and reaction composition—researchers can achieve robust amplification efficiencies between 90% and 110%. This level of precision, coupled with rigorous quality controls and appropriate normalization using both exogenous spike-ins and stable endogenous miRNAs [13] [17], ensures that the resulting data are reliable and suitable for biomarker discovery, validation, and eventual translation into clinical applications.
The accuracy of Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR) for plasma microRNA (miRNA) quantification depends critically on proper data normalization. Variations in sample collection, RNA extraction efficiency, and reverse transcription can introduce significant technical biases that obscure true biological signals. For miRNA research in plasma—a complex, acellular environment lacking traditional housekeeping genes—the choice between endogenous control miRNAs and exogenous spike-in controls represents a fundamental methodological decision. This protocol examines advanced strategies for both approaches, providing researchers with a framework to implement robust normalization tailored to their experimental conditions, thereby ensuring data reliability and reproducibility in biomarker discovery and validation studies.
Theoretical Foundation: Normalization Approaches and Their Applications
Endogenous Controls: Stability in Biological Context
Endogenous controls are naturally occurring miRNAs present in plasma that exhibit stable expression across the experimental conditions being studied. Their key advantage lies in undergoing the same technical variations as target miRNAs throughout the entire experimental workflow, from RNA extraction to PCR amplification. Ideal endogenous normalizers should demonstrate consistent expression unaffected by the biological variables under investigation [25]. For instance, in COVID-19 research, hsa-miR-205-3p was identified as a stable normalizer when comparing infected versus non-infected individuals, though it proved unsuitable for severity comparisons [25]. Similarly, research on Alzheimer's disease populations identified seven stable miRNA normalizers that remained consistent across age, sex, and disease status [71].
Spike-In Controls: Technical Process Monitoring
Exogenous spike-in controls are synthetic RNAs, such as the widely used cel-miR-39 from C. elegans, added to samples at known concentrations at specific points in the workflow. These controls primarily monitor technical efficiency rather than biological relevance. As outlined in Bio-protocol exchanges, cel-miR-39 is typically added after the addition of QIAzol lysis reagent during RNA extraction, enabling normalization for variations in RNA isolation efficiency but not for losses during earlier steps [19]. Advanced protocols recommend "double spike-in" controls added at both the RNA isolation and reverse transcription steps to monitor the efficiency of multiple technical processes [71].
Table 1: Comparison of Normalization Approaches for Plasma miRNA Studies
| Feature | Endogenous Controls | Spike-In Controls |
|---|---|---|
| Basis of Selection | Stable expression across study groups | Synthetic, non-human sequences |
| Monitors Variation In | Entire workflow including biological factors | Technical steps after addition |
| Optimal Application | Case-control studies with stable normalizers | Absolute quantification; efficiency controls |
| Key Advantages | Experience same biological matrix effects; no additional cost | Known concentration; unaffected by human biology |
| Key Limitations | Context-dependent stability; requires validation | Doesn't account for pre-addition losses; additional cost |
| Example Molecules | hsa-miR-205-3p (COVID-19) [25]; 7-miRNA panel (Alzheimer's) [71] | cel-miR-39 [19] |
Experimental Protocols: Implementation Workflows
Protocol 1: Systematic Identification of Endogenous Normalizers
This protocol outlines a comprehensive approach for identifying and validating endogenous normalizers specific to a research context, adapted from COVID-19 and Alzheimer's disease studies [25] [71].
Step 1: Candidate Selection
- Begin with high-throughput screening (RNA-Seq or large-scale PCR arrays) of candidate miRNAs in a representative subset of samples (e.g., 5-10 per group)
- Apply selection criteria: fold regulation ≈ 1.0, p-value > 0.990 in inter-group comparisons [25]
- Prioritize miRNAs discovered longer ago with more established literature
- Include known stable miRNAs from related fields (e.g., miR-16, miR-191) as comparators despite potential context-specific limitations [25]
Step 2: Technical Validation
- Conduct RT-qPCR validation in full cohort (minimum n=40 per group recommended)
- Use miRCURY or TaqMan Advanced miRNA cDNA Synthesis kits per manufacturer protocols
- Perform stability analysis using RefFinder, which integrates geNorm, NormFinder, BestKeeper, and comparative ΔCq methods [25] [72]
- Apply the novel BestmiRNorm algorithm for assessing up to 11 potential normalizers simultaneously when available [71]
Step 3: Stability Confirmation
- Verify candidate normalizers show no significant expression differences (p > 0.05) between experimental groups
- Confirm consistent expression in relevant biological fractions (e.g., plasma-derived exosomes) when applicable [3]
- Validate normalizer stability across all technical replicates and experimental batches
Protocol 2: Implementation of Spike-In Controls
This protocol details the proper implementation of exogenous spike-in controls at multiple points in the workflow to monitor technical efficiency [19] [71].
Step 1: Strategic Spike-In Addition
- Add first spike-in (e.g., cel-miR-39) immediately after denaturation with QIAzol or similar lysis reagent to monitor RNA isolation efficiency [19]
- Add second spike-in after RNA isolation but before reverse transcription to monitor cDNA synthesis efficiency
- Use consistent, empirically determined concentrations across all samples (typically 1-5 fmol/μL)
- Verify spike-in concentrations do not saturate amplification or interfere with endogenous miRNA detection
Step 2: Efficiency Calculation and Application
- Extract RNA using miRNeasy Serum/Plasma Advanced Kit or equivalent
- Perform reverse transcription with High-Capacity RNA-to-cDNA kit
- Calculate isolation efficiency: (recovered spike-in 1 / added spike-in 1)
- Calculate RT efficiency: (recovered spike-in 2 / added spike-in 2)
- Apply correction factors to target miRNA Cq values based on efficiency calculations
Step 3: Quality Control and Exclusion Criteria
- Establish acceptable efficiency ranges (typically 70-130%) during validation
- Exclude samples with efficiency deviations beyond predetermined thresholds
- For hemolysis assessment, use absorbance-based detection or miR-23a-3p/miR-451a ratio (ΔCq < 7 indicates acceptable quality) [71]
- Document all efficiency values and exclusion decisions for reporting transparency
Protocol 3: Hybrid Normalization Strategy
For maximum robustness, implement a hybrid approach combining endogenous and exogenous controls, particularly recommended for clinical biomarker studies [25] [71] [12].
Step 1: Parallel Processing
- Process all samples with dual spike-in controls as in Protocol 2
- Simultaneously measure 3-5 validated endogenous normalizers as in Protocol 1
- Include both types of controls in all RT-qPCR runs
Step 2: Data Integration and Consistency Checking
- Normalize data using both approaches independently
- Compare results for consistency between normalization methods
- Resolve discrepancies by investigating technical artifacts or biological factors
- Use geometric mean of multiple stable endogenous normalizers when possible [71]
Step 3: Final Normalization Application
- Prioritize endogenous normalization for case-control comparisons when stable normalizers exist
- Use spike-in normalization for absolute quantification or when endogenous normalizers show variability
- Apply efficiency corrections from spike-ins to endogenously normalized data for highest accuracy
- Document all normalization decisions in methods section
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for Plasma miRNA Normalization Studies
| Reagent/Kit | Primary Function | Application Notes |
|---|---|---|
| miRNeasy Serum/Plasma Advanced Kit (Qiagen) | miRNA extraction from plasma/serum | Compatible with spike-in addition; high recovery of small RNAs |
| TaqMan Advanced miRNA cDNA Synthesis Kit (Applied Biosystems) | cDNA synthesis for miRNA quantification | Poly(A) tailing and adapter ligation for mature miRNA detection |
| miRCURY Exosome Serum/Plasma Kit (Qiagen) | Exosome isolation from plasma | Enables fraction-specific miRNA profiling [3] |
| cel-miR-39 synthetic RNA | Exogenous spike-in control | C. elegans miRNA not found in human samples; add after lysis |
| HTG EdgeSeq miRNA WTA | High-throughput miRNA profiling | Profiles 2083 miRNAs; useful for initial normalizer screening [12] |
| RefFinder Web Tool | Stability analysis of candidate normalizers | Integrates multiple algorithms (geNorm, NormFinder, BestKeeper) [25] |
Data Interpretation and Troubleshooting
Stability Assessment and Validation
When evaluating candidate normalizers, stability should be quantitatively assessed rather than assumed. The geNorm algorithm calculates a stability measure (M) where lower values indicate greater stability, with M < 1.5 generally considered acceptable [3]. For the NORMA-Gene method, which uses a least-squares regression approach without reference genes, the key metric is reduction in variance of target genes across experimental samples compared to other normalization methods [73]. When applying spike-in controls, establish coefficient of variation thresholds (typically <5% for intra-assay, <10% for inter-assay) for technical acceptance.
Troubleshooting Common Normalization Issues
High Variation in Endogenous Controls: If candidate normalizers show unexpected variability, first verify sample quality by checking hemolysis indicators (miR-451a/miR-23a-3p ratio or absorbance measurements) [71]. Consider expanding the panel of candidate normalizers or implementing a global mean normalization approach if sufficient miRNA targets are measured.
Inconsistent Spike-In Recovery: Large variations in spike-in recovery between samples may indicate issues with pipetting accuracy, RNA isolation technique, or RT inhibition. Implement additional spike-in controls at different workflow stages to pinpoint the problematic step and ensure consistent sample volumes during processing.
Discordant Results Between Methods: When endogenous and spike-in normalization yield conflicting results, investigate potential biological factors affecting endogenous normalizers and verify spike-in addition timing and concentrations. Consider whether study groups differ in factors that might affect global miRNA profiles (e.g., platelet count, inflammatory status) that could impact endogenous normalizer stability.
The choice between endogenous controls and spike-ins depends on study objectives, sample types, and available resources. For case-control studies investigating relative expression differences, endogenous normalizers validated in the specific biological context provide the most biologically relevant normalization, as demonstrated in MRONJ research where miR-483-5p, miR-92-5p, miR-628-3p, and miR-486-5p were identified as disease-specific biomarkers [3]. For absolute quantification or studies where no stable endogenous normalizers can be identified, spike-in controls offer technical precision. The most robust approach combines both strategies, using spike-ins to monitor technical efficiency while employing endogenous controls for biological normalization, thereby maximizing data reliability in plasma miRNA research for both basic science and clinical applications.
The quantification of microRNAs (miRNAs) in plasma using reverse transcription quantitative polymerase chain reaction (RT-qPCR) is a cornerstone of non-invasive biomarker research for conditions ranging from cancer to infectious diseases [20] [25]. However, the precision of this powerful technique can be compromised by pre-analytical variables, instrument performance, and software analysis parameters, introducing variability that threatens the reproducibility and clinical translation of findings. This application note details the principal sources of this variability and provides validated protocols to ensure consistent platform performance in miRNA research.
A primary technical challenge stems from the low abundance and short length of miRNA molecules, which demands exceptional sensitivity from detection platforms [74]. Furthermore, normalization strategies—a critical step for accurate quantification—lack universal standardization, with researchers often debating between endogenous miRNAs and exogenous spike-ins [25]. The recent emergence of digital PCR (dPCR) offers an alternative with absolute quantification but introduces new considerations for platform integration [75]. This document provides a structured framework to navigate these challenges, enabling researchers to produce robust and reliable data.
Pre-Analytical and Analytical Variability
The journey of a plasma sample from collection to miRNA quantification is fraught with potential sources of error. A key finding from recent stability studies is that miRNA profiles in plasma and serum demonstrate remarkable stability when stored at room temperature for up to 24 hours, with over 99% of the miRNA profile remaining unchanged even when blood draw tubes were left at room temperature for 6 hours prior to processing [20]. This resilience is advantageous for handling in routine clinical settings. Despite this, the reverse transcription step is particularly sensitive and a significant contributor to technical variation, as it is influenced by factors like salt, alcohol, or phenol residues in the sample [76].
Instrument and Software Variability
Different quantification platforms can introduce systematic bias. RT-qPCR relies on relative quantification based on standard curves, making the generation and application of these curves a critical point of variability. Key parameters such as amplification efficiency, slope, and y-intercept can fluctuate between experiments [76]. This inter-assay variability means that a standard curve generated in one run may not be directly applicable to another, potentially compromising the reliability of results. Studies have shown that variability differs between viral targets; for instance, the SARS-CoV-2 N2 gene exhibited a coefficient of variation (CV) of 4.38–4.99%, while Norovirus GII showed significant inter-assay variability in efficiency [76]. Adherence to the MIQE guidelines (Minimum Information for Publication of Quantitative Real-Time PCR Experiments) is essential for mitigating these issues and ensuring the transparency and reproducibility of published data [76].
Digital PCR (dPCR) is gaining traction as it provides absolute quantification without the need for standard curves, thereby eliminating a major source of variability [75]. However, its transition to mainstream use involves challenges related to equipment cost, workflow throughput, and data-analysis learning curves [75].
Table 1: Key Sources of Technical Variability in miRNA Quantification
| Variability Category | Specific Source | Impact on Data |
|---|---|---|
| Pre-Analytical | Sample Collection & Processing | Potential miRNA degradation or hemolysis (though miRNAs are generally stable [20]) |
| RNA Extraction Efficiency | Inconsistent yield and purity affecting downstream reactions | |
| Analytical | Reverse Transcription (RT) Efficiency | Major source of variation due to reaction inhibitors and enzyme fidelity [76] |
| PCR Amplification Efficiency | Affected by primer design, reagent quality, and thermocycler calibration | |
| Platform & Software | Standard Curve Construction (qPCR) | Inter-assay variability in slope, efficiency, and y-intercept [76] |
| Threshold Setting & Data Analysis | Software algorithms and manual threshold settings can alter Cq/CT values |
Established Protocols for Minimizing Variability
Protocol: Reliable Plasma miRNA Quantification via RT-qPCR
This protocol is designed to control variability throughout the process of miRNA quantification from plasma.
I. Sample Preparation and RNA Extraction
- Sample Collection: Collect whole blood in K₂EDTA tubes (for plasma) or clotting tubes (for serum). For plasma, centrifuge at 1200×g for 10 minutes at room temperature. Carefully collect the top layer and perform a second centrifugation at 1500×g for 5 minutes to remove residual cells [20].
- RNA Extraction: Isolate miRNA using a dedicated kit, such as the Qiagen miRNeasy Serum/Plasma Kit. Incorporate a synthetic spike-in control (e.g., cel-miR-39) during the lysis step to monitor extraction efficiency and correct for technical variation [25] [19]. Elute RNA in a reduced volume (e.g., 28 µL) to increase concentration.
II. Reverse Transcription and qPCR Setup
- cDNA Synthesis: Use a robust cDNA synthesis kit (e.g., Applied Biosystems High-Capacity RNA-to-cDNA Kit). Maintain consistent reaction conditions and volumes across all samples.
- qPCR Reaction: Perform reactions in triplicate using TaqMan MicroRNA Assays and a universal probe supermix (e.g., iTaq Universal Probes Supermix from Bio-Rad) [20]. A well-defined thermocycling protocol is critical:
- Initial Denaturation: 95°C for 10 minutes
- 40–45 Cycles of:
- Denaturation: 94°C for 15 seconds
- Annealing/Extension: 60°C for 1 minute [19]
III. Data Normalization and Analysis
- Normalization Strategy: Normalize data using the stable endogenous normalizer identified for your specific disease context. For COVID-19, for example, hsa-miR-205-3p has been validated as a stable normalizer in plasma, whereas commonly used genes like miR-16 may not be stable [25].
- Quantification: Calculate relative expression using the 2^–ΔΔCq method, incorporating values from the stable normalizer and the spike-in control [19].
Protocol: Implementing Quality Control with Standard Curves
To address instrument and inter-assay variability in RT-qPCR, the following quality control procedure is recommended.
I. Standard Curve Generation
- For each experiment and viral target, include a standard curve based on at least a four-point serial dilution of quantitative synthetic RNA with known concentrations [76].
- Prepare a sufficient number of aliquots of the synthetic RNA standard to ensure each experiment uses an aliquot that has undergone only a single freeze-thaw cycle, preventing degradation-induced variability.
II. Data Acquisition and Threshold Setting
- Run the standard curve dilutions in duplicate on the same plate as the experimental samples.
- To ensure comparability across runs, manually set a fixed threshold for fluorescence rather than relying solely on the instrument's automated setting. The software-generated threshold (e.g., 10x the standard deviation of the baseline) can be slightly adjusted to a consistent, practical decimal value for all experiments (e.g., 0.04) [76].
III. Validation and Acceptance Criteria
- Calculate the amplification efficiency (E) for each run using the formula: E = (10^(–1/slope)) - 1 [76].
- Only accept experimental results from runs where the standard curve meets pre-defined quality metrics, such as an amplification efficiency between 90–110% and a correlation coefficient (R²) > 0.99.
The following workflow summarizes the critical steps and decision points in these protocols to ensure consistency and identify sources of variability.
The Scientist's Toolkit: Essential Research Reagents & Materials
Table 2: Key Research Reagent Solutions for Plasma miRNA Analysis
| Item | Function/Application | Example Products/Candidates |
|---|---|---|
| RNA Extraction Kit | Isolation of high-quality small RNAs from plasma/serum | Qiagen miRNeasy Serum/Plasma Kit [20] |
| Synthetic Spike-in miRNA | Exogenous control for normalization of extraction efficiency and technical variation | cel-miR-39 [25] [19] |
| Stable Endogenous Normalizers | Biological reference genes for data normalization | hsa-miR-205-3p (validated for COVID-19) [25]; miR-15b, miR-16, miR-21 (shown to be stable in healthy plasma [20]) |
| Reverse Transcription Kit | Synthesis of cDNA from miRNA templates | Applied Biosystems High-Capacity RNA-to-cDNA Kit [20] |
| qPCR Assay System | Specific detection and amplification of target miRNAs | TaqMan MicroRNA Assays, SYBR Premix Ex Taq [20] [19] |
| Quantitative Synthetic RNA | Generation of standard curves for absolute or relative quantification | Commercial synthetic RNAs (e.g., from ATCC) [76] |
Ensuring consistent performance across instruments and software is not merely a technical exercise but a fundamental requirement for generating credible, reproducible miRNA data that can accelerate biomarker discovery and drug development. The protocols detailed herein—emphasizing rigorous quality control, the use of stable normalizers, and standardized data analysis—provide a actionable path forward. As the field evolves with new technologies like dPCR and AI-driven analytics [74] [75], the principles of meticulous validation and transparency remain paramount. By systematically addressing the documented sources of variability, researchers can significantly enhance the reliability of their RT-qPCR workflows and strengthen the foundation for future clinical applications.
Troubleshooting Poor Yield, Low Sensitivity, and Inconsistent Replicates
The quantification of circulating microRNAs (miRNAs) in plasma using RT-qPCR presents a significant opportunity for non-invasive biomarker discovery in clinical research and drug development. miRNAs are short (~22 nucleotides), non-coding RNA molecules that play a critical role in post-transcriptional gene regulation and exhibit remarkable stability in circulation [77]. Their unique ability to simultaneously modulate multiple gene expressions and their tissue-specific expression patterns have positioned them as key players in understanding complex biological mechanisms and disease pathogenesis, particularly in cancer [77]. However, the technical challenges associated with their detection—including poor yield, low sensitivity, and inconsistent replicates—have hindered their reliable implementation in research and clinical applications.
These challenges stem from multiple factors: the inherently low concentration of miRNAs in plasma, the presence of PCR inhibitors, inefficient reverse transcription due to the short length of miRNA molecules, and suboptimal normalization strategies [71] [68]. This application note systematically addresses these critical troubleshooting areas within the context of a broader thesis on RT-qPCR protocol standardization for miRNA quantification in plasma research, providing researchers with validated solutions to enhance data reliability and reproducibility.
Troubleshooting Poor Yield
RNA Extraction Optimization
Poor miRNA yield from plasma samples often originates from suboptimal RNA extraction efficiency. The selection of appropriate extraction methodologies is crucial for maximizing recovery from these challenging, low-input samples.
Table 1: Comparison of miRNA Extraction Kits for Plasma Samples
| Kit Name | Principle | Recommended Plasma Volume | Performance with Low-Input Samples | Key Considerations |
|---|---|---|---|---|
| miRNeasy Serum/Plasma Kit (Qiagen) | Silica-membrane based spin column | 100-200 µL [68] | Outperforms other kits with limited plasma quantity [78] | Compatible with low-volume paediatric samples [68] |
| MagMAX miRVana Total Isolation Kit (Thermo Fisher) | Magnetic bead-based purification | 100-200 µL [68] | Lower yield compared to miRNeasy in validation studies [68] | Amenable to automation; faster processing times |
| Phenol-Chloroform (Trizol) Methods | Organic extraction | Variable | Selective loss of small RNAs with low GC content [68] | Time-consuming; risk of contaminant carryover |
A modified protocol for the miRNeasy Serum/Plasma Kit has demonstrated improved miRNA output for downstream analyses [78]. For low-volume paediatric samples (100µL plasma), this kit produced higher miRNA library concentrations (0-1.42 ng/µL average) compared to magnetic bead-based methods (0-0.11 ng/µL average) [68]. When working with limited sample volumes, ensure consistent input volumes across comparisons, as studies show that varying plasma input volumes (100µL vs. 200µL) demonstrate no significant differences in yield, allowing for volume conservation without compromising data integrity [68].
Reverse Transcription Efficiency
The short length of miRNA molecules presents unique challenges for reverse transcription. Conventional stem-loop RT primers often have limited binding efficiency and may not effectively capture the target miRNA sequence, potentially compromising detection sensitivity [77]. To address this, novel approaches such as the Reverse Transcription-Hairpin Occlusion System (RT-HOS) have been developed, which allows reverse transcription at higher temperatures, thereby enhancing specificity and accelerating the reaction [77].
For routine analysis, the TaqMan Advanced miRNA cDNA Synthesis Kit includes a preamplification step designed to enrich target sequences prior to detection, ensuring both optimal reverse transcription efficiency and consistent cDNA quality for downstream applications [53]. When cDNA yield is low, consider increasing the amount of RNA input into your reverse transcription reaction (if possible) and using a fixed input volume when RNA concentration is below detection thresholds, as recommended for serum samples [53].
Addressing Low Sensitivity
Assay Design and Detection Chemistry
Low sensitivity in miRNA detection frequently results from suboptimal assay design and inappropriate detection chemistry selection. The extremely short length of miRNA fragments—approximately the same length as conventional PCR primers—makes traditional multiplex RT-qPCR approaches particularly challenging [77].
Table 2: Comparison of miRNA Detection Methodologies
| Methodology | Principle | Limit of Detection | Advantages | Limitations |
|---|---|---|---|---|
| Stem-Loop RT-qPCR | Specific stem-loop RT primers followed by qPCR | High | High specificity; gold standard | Two-step process; time-consuming; risk of sample loss |
| Poly-A Tailing RT-qPCR | Enzymatic poly-A tailing followed by oligo-dT RT | Lower than stem-loop | Does not require specific RT primers | Variable extension efficiency; sequence-specific bias |
| One-Pot Methods (HOM/TOM-qPCR) | Single-step multiplex using RT-HOS [77] | 7.5 × 101 to 7.5 × 108 copies/reaction [77] | Reduced time; minimal cross-contamination; high specificity | Novel method requiring validation |
| Digital PCR (dPCR) | Absolute quantification by sample partitioning | Superior sensitivity for low-abundance miRNAs [53] | Absolute quantification without standard curves; resistant to inhibitors | Higher cost; specialized equipment required |
Recent innovations include one-pot, one-step miRNA multiplex RT-qPCR methods mediated by RT-HOS, which can be driven by either High-Fidelity DNA polymerase or Taq DNA polymerase [77]. These methods demonstrate a wide linear dynamic range from 7.5 × 10^8 to 7.5 × 10^1 copies/reaction while maintaining excellent specificity for distinguishing closely related miRNA sequences [77]. For applications requiring maximum sensitivity, digital PCR (dPCR) offers a robust solution, with demonstrated superior sensitivity particularly for detecting low-abundance miRNAs compared to qRT-PCR [53].
Sensitivity Enhancement Strategies
When sensitivity remains inadequate despite optimized assay design, consider these technical adjustments:
- Increase template input: Increase the amount of cDNA in your qPCR reaction (up to 20% by volume maximum) [79].
- Alternative reverse transcription: Try a different reverse transcription kit, such as the SuperScript VILO Master Mix, for the highest cDNA yield possible [79].
- Preamplification strategies: Implement a preamplification step before main qPCR to enrich target sequences, particularly crucial for low-abundance targets [53].
- Transition to dPCR: For critically important low-abundance targets, consider adopting digital PCR, which has demonstrated improved detection of miRNAs present at low concentrations in serum [53].
Resolving Inconsistent Replicates
Normalization Strategies
Inconsistent replicates often stem from inappropriate normalization methods. For circulating miRNAs in plasma, standard normalizers such as GAPDH or β-actin are not physiologically relevant [71]. Proper normalization requires exogenous spike-in controls and stable endogenous normalizers.
Exogenous Spike-In Controls: Incorporate synthetic miRNAs (e.g., cel-miR-39, UniSp6) during the RNA isolation phase to monitor extraction efficiency and reverse transcription variability [71] [68]. These controls account for technical variability throughout the workflow.
Endogenous Normalizers: A recent large-scale study (140 subjects) identified 7 stable normalizers for aging populations and Alzheimer's disease research: let-7d-5p, let-7g-5p, let-7i-5p, miR-103a-3p, miR-107, miR-16-5p, and miR-532-5p [71]. The study introduced BestmiRNorm, a novel Python-based method for identifying optimal normalizers from a panel of candidates, enabling assessment of up to 11 potential normalizers with computational efficiency [71].
Hemolysis Assessment: Implement absorbance-based haemoglobin detection or the miR-23a-3p/miR-451a ratio method (ΔCq < 7 indicates minimal haemolysis) to exclude compromised samples [71].
Technical Precision and Automation
Inconsistent pipetting represents a major source of technical variability in RT-qPCR workflows, particularly when handling the small volumes typical of miRNA reactions [80].
- Pipette Calibration: Regularly calibrate pipettes, use positive-displacement pipettes and filtered tips, and ensure proper vertical pipetting technique during solution aspiration [81].
- Automated Liquid Handling: Systems like the I.DOT Liquid Handler minimize cross-contamination through a closed, tipless system and can accurately handle volumes as low as 4 nL, ensuring consistent Ct values across experimental replicates [80].
- Master Mix Preparation: Prepare a single master mix for all replicates of each assay to minimize tube-to-tube variation and ensure consistent reagent volumes across reactions [80].
- Threshold Setting: Allow instrument software to automatically set a proper threshold, then verify that it lies significantly higher than baseline fluorescence and below the plateau phase, typically at the mid-point of the exponential phase [81].
Integrated Workflow and Reagent Solutions
Comprehensive Troubleshooting Workflow
Research Reagent Solutions
Table 3: Essential Reagents and Kits for miRNA RT-qPCR
| Reagent/Kits | Specific Product Examples | Function in Workflow | Key Features |
|---|---|---|---|
| RNA Extraction Kits | miRNeasy Serum/Plasma Kit (Qiagen) [78] | miRNA isolation from plasma | Optimized for low-volume samples; high recovery efficiency |
| Reverse Transcription Kits | TaqMan Advanced miRNA cDNA Synthesis Kit (Thermo Fisher) [53] | cDNA synthesis from miRNA | Includes preamplification step; universal RT for all miRNAs |
| qPCR Master Mixes | EXPRESS SYBR GreenER qPCR SuperMix (Invitrogen) [82] | Fluorescent detection | Compatible with SYBR Green chemistry; includes UDG contamination control |
| Spike-In Controls | UniSp6 (Qiagen) [68], cel-miR-39 | Process monitoring | Synthetic miRNAs added pre-extraction; controls for technical variability |
| Normalization Panels | let-7d-5p, let-7g-5p, let-7i-5p, miR-103a-3p, miR-107, miR-16-5p, miR-532-5p [71] | Data normalization | 7 stable normalizers validated in aging and AD populations |
| Automation Systems | I.DOT Liquid Handler (Dispendix) [80] | Liquid handling automation | Non-contact dispensing; handles volumes as low as 4 nL; reduces cross-contamination |
Successful miRNA quantification in plasma requires a systematic approach to troubleshooting poor yield, low sensitivity, and inconsistent replicates. By implementing optimized RNA extraction protocols, adopting advanced detection methodologies such as one-pot RT-qPCR or dPCR, and employing rigorous normalization strategies with appropriate endogenous normalizers and spike-in controls, researchers can significantly enhance the reliability of their miRNA data. Automated liquid handling systems further contribute to reproducibility by minimizing technical variability. Through the comprehensive application of these strategies, circulating miRNA profiling can achieve the robustness required for meaningful biomarker discovery and validation in both basic research and clinical applications.
Ensuring Reproducibility: Method Validation and Cross-Platform Comparisons
The development of robust Acceptance Criteria for Sensitivity, Specificity, and Precision is paramount for the validation of RT-qPCR protocols targeting circulating microRNAs (miRNAs) in plasma. Circulating miRNAs hold significant promise as minimally invasive biomarkers for a range of ageing-related diseases, including Alzheimer's disease (AD) [20] [13]. Unlike longer RNA transcripts, miRNAs are short oligonucleotides that demonstrate remarkable stability in biofluids, being packaged within exosomes or complexed with proteins, which protects them from degradation by ubiquitous RNases [20]. However, the transition of miRNA biomarkers from research to clinical diagnostics has been hampered by a lack of standardization, particularly in the RT-qPCR normalization step, leading to inconsistent data across studies [13]. This document outlines detailed application notes and protocols to establish rigorous, reproducible acceptance criteria, framed within the context of a broader thesis on miRNA quantification.
Defining Acceptance Criteria for miRNA Biomarker Assays
The following criteria should be established a priori to validate the performance of an RT-qPCR assay for miRNA detection in plasma.
| Criterion | Definition | Target Value | Application in miRNA RT-qPCR |
|---|---|---|---|
| Sensitivity | The ability of an assay to correctly identify true positives. | ≥ 85% | The probability that the assay will correctly detect a differentially expressed miRNA in a patient's plasma sample when the disease is truly present. |
| Analytical Sensitivity | The lowest concentration of an analyte that can be reliably detected. | Dependent on the specific miRNA and the limit of detection (LOD) of the assay. | Can be enhanced by using nanomaterial-engineered biosensors which provide amplified signals and overcome detection barriers [83]. |
| Specificity | The ability of an assay to correctly identify true negatives. | ≥ 90% | The probability that the assay will correctly yield a negative result for a control plasma sample from a healthy individual. Ensures the miRNA signal is specific to the disease state and not due to cross-reactivity or haemolysis. |
| Precision | The closeness of agreement between independent measurements of the same sample under stipulated conditions. | CV < 10-15% | Encompasses repeatability (within-run precision) and reproducibility (between-run, between-operator, between-laboratory precision). Critical for confirming that observed Cq value variations are biologically significant and not technical noise [13]. |
Detailed Experimental Protocol for miRNA Analysis in Plasma
This protocol is optimized for assessing the key acceptance criteria in the context of ageing-related diseases, incorporating steps to control pre-analytical variables.
Sample Collection and Quality Control
- Blood Collection: Draw whole blood into K2EDTA tubes (for plasma) or clotting tubes (for serum). For plasma, centrifuge at 1200×g for 10 minutes at room temperature immediately after collection [20].
- Plasma Processing: Carefully collect the top layer (plasma) and perform a second centrifugation at 1500×g for 5 minutes to remove residual cells [20].
- Haemolysis Assessment: Haemolysis is a critical confounder for circulating miRNA profiles. Assess sample quality using one of two methods:
- Absorbance-based method: Measure absorbance at 414 nm (peak for haemoglobin) and 375 nm (reference). An elevated 414/375 nm ratio indicates haemolysis [13].
- miRNA Delta Cq method: Perform RT-qPCR for miR-23a-3p (plasma marker) and miR-451a (RBC marker). A ΔCq (Cq~miR-23a-3p~ – Cq~miR-451a~) < 7 suggests the sample is clear of significant haemolysis [13].
- Stability Tests: Data indicates miRNAs (e.g., miR-15b, miR-16, miR-21, miR-24, miR-223) are stable in plasma and serum for up to 24 hours at room temperature or on ice, making them robust to routine clinical handling variability [20].
miRNA Isolation and Reverse Transcription
- Isolation: Use commercial kits specifically designed for miRNA from serum/plasma (e.g., Qiagen miRNeasy Serum/Plasma Kit). Elute in a reduced volume (e.g., 28 µL) to concentrate the sample [20].
- Spike-in Controls: Incorporate exogenous spike-in controls (e.g., C. elegans miR-39) during the isolation step to monitor and correct for variations in miRNA extraction efficiency and subsequent reverse transcription [13].
Quantitative PCR (qPCR) and Data Normalization
- qPCR Setup: Perform all reactions in triplicate using probe-based chemistry (e.g., TaqMan MicroRNA Assays) [20].
- Instrument Consistency: Perform all steps of RT-qPCR analysis, including normalization, using the same machine and software for the entire study. Variability between machines (e.g., StepOnePlus vs. 7900HT) and analysis software (e.g., SDS vs. ExpressionSuite) can significantly alter Cq values and impact acceptance criteria [13].
- Normalization: This is the most critical step for achieving accurate precision. The use of a single housekeeping gene is not recommended.
- Strategy: Use a global mean normalization approach when a large number of miRNAs are analyzed. For smaller-scale RT-qPCR studies, identify and use a combination of stable endogenous normalizers [13].
- Verified Normalizers: For studies on an ageing population (including Alzheimer's patients), a panel of 7 stable normalizers has been identified and verified using a novel method called BestmiRNorm [13]. This method, developed in Python, allows for the assessment of up to 11 potential normalizers to identify the most stable ones for a given experimental context.
Experimental Workflow for Protocol Establishment
The following diagram illustrates the logical workflow for establishing and validating the RT-qPCR protocol for circulating miRNA analysis.
The Scientist's Toolkit: Key Research Reagent Solutions
The following reagents and controls are essential for ensuring the sensitivity, specificity, and precision of the miRNA RT-qPCR assay.
| Reagent / Material | Function / Explanation | Example Product / Citation |
|---|---|---|
| miRNA Serum/Plasma Kit | Optimized for isolation of small RNAs from low-volume, cell-free biofluids. | Qiagen miRNeasy Serum/Plasma Kit [20] |
| Spike-in Control miRNAs | Exogenous miRNAs (e.g., from C. elegans) added to monitor efficiency of isolation and reverse transcription; critical for precision. | e.g., cel-miR-39 [13] |
| Haemolysis Detection Assay | Controls for sample quality; assesses potential contamination with RBC-specific miRNAs that could confound results and affect specificity. | RT-qPCR for miR-23a-3p vs. miR-451a [13] |
| TaqMan MicroRNA Assays | Provide high-specificity primers and probes for individual miRNA targets, minimizing off-target amplification. | Applied Biosystems [20] |
| Stable Normalizer Panel | A combination of endogenous miRNAs verified to be stable across the experimental cohort, essential for precise ΔΔCq calculations. | Panel of 7 normalizers for ageing/AD studies [13] |
| High-Capacity cDNA Kit | Reverse transcription kit optimized for converting miRNA into stable cDNA for qPCR amplification. | Applied Biosystems High-Capacity RNA-to-cDNA Kit [20] |
Data Analysis Pathway for Acceptance Criteria
The final step involves a rigorous data analysis pathway to apply the defined acceptance criteria, as shown in the following workflow.
Implementing Good Laboratory Practice (GLP) Principles for miRNA Analysis
MicroRNAs (miRNAs) have emerged as promising biomarkers for various diseases, including cancer, neurodegenerative disorders, and metabolic conditions [84] [13]. Their stability in circulation and sensitivity to pathological changes make them particularly valuable for diagnostic and prognostic applications. However, the transition of miRNA analysis from research settings to clinical applications requires stringent implementation of Good Laboratory Practice (GLP) principles to ensure data reliability, reproducibility, and quality [13]. This document outlines standardized protocols and application notes for miRNA quantification in plasma, framed within a broader thesis on RT-qPCR methodology. The procedures described herein address critical challenges in miRNA analysis, including pre-analytical variables, normalization strategies, and quality control measures, providing researchers with a comprehensive framework for generating robust, reproducible data in compliance with GLP standards.
Quantitative Data on miRNA Analysis Performance
Table 1: Technical Performance Characteristics of miRNA RT-qPCR Analysis
| Parameter | Performance Characteristic | Experimental Conditions | Reference |
|---|---|---|---|
| Lower Limit of Quantification (LLOQ) | 10² copies/µL for most miRNAs | Probe-based RT-qPCR with pre-amplification | [85] |
| Technical Variation | < 3-fold | Absolute quantification with standardized protocol | [85] |
| Sample Volume | 100-200 µL plasma/serum | Optimal detection range for low-abundance miRNAs | [85] [16] |
| Haemolysis Assessment | ΔCq (miR-23a-3p – miR-451a) < 7 | Acceptable haemolysis threshold | [13] |
| miRNA Concentration Range | 0.25-1.0 ng/µL | Extracted from 100 µL plasma/serum | [16] |
| Detection Without Pre-amplification | miR-122, miR-133a, miR-192 | Higher abundance miRNAs | [85] |
| Detection Requiring Pre-amplification | miR-1, miR-206, miR-499a | Lower abundance miRNAs | [85] |
Table 2: Stable Normalizers for miRNA RT-qPCR in Ageing Populations
| Normalizer miRNA | Stability Characteristics | Population Validation | Reference |
|---|---|---|---|
| Panel of 7 miRNAs | Stable across ageing populations | 140 subjects (healthy & Alzheimer's patients) | [13] |
| cel-miR-238 | Spike-in control for normalization | Cynomolgus monkey plasma samples | [85] |
| cel-miR-39-3p | Spike-in control for extraction efficiency | Feline plasma/serum samples | [16] |
Experimental Protocols
Sample Collection and Processing Protocol
Principle: Standardized sample collection and processing is critical for minimizing pre-analytical variation in miRNA analysis [13]. Consistent procedures ensure sample quality and reproducibility.
Materials:
- K₃EDTA-containing tubes (avoid heparin due to PCR inhibition)
- Refrigerated centrifuge
- Low-binding microtubes
- -80°C freezer
Procedure:
- Blood Collection: Collect blood via venipuncture (minimum 0.5 mL) into K₃EDTA-containing tubes [85].
- Initial Processing: Place samples immediately on ice and process for plasma isolation within 2 hours of collection [85].
- Plasma Separation:
- Secondary Centrifugation: Centrifuge at 16,000 × g for 5-10 minutes at 4°C to remove cell debris and residual platelets [85] [16].
- Aliquoting: Aliquot 200 µL of supernatant into fresh low-binding microtubes [85]. Use exact volumes for consistency.
- Storage: Store aliquots at -80°C until RNA extraction. Avoid repeated freeze-thaw cycles [16].
Quality Control:
- Assess haemolysis using absorbance measurement at 414 nm [13].
- Alternatively, use ΔCq (miR-23a-3p – miR-451a) with threshold <7 for acceptable haemolysis [13].
RNA Extraction and Quality Control Protocol
Principle: Efficient RNA isolation with minimal degradation is essential for accurate miRNA quantification. Incorporation of spike-in controls monitors technical variation [85] [13].
Materials:
- miRNeasy Mini Kit or equivalent
- Chloroform
- Synthetic cel-miR-238 or cel-miR-39-3p
- Ethanol (100% and 70-80%)
- RNase-free water
- Fluorometer with microRNA assay capability
Procedure:
- Sample Thawing: Thaw frozen plasma/serum samples on ice [85].
- Lysis:
- Add 5 volumes (1000 µL for 200 µL sample) of lysis reagent containing phenol and guanidine isothiocyanate [85].
- Mix vigorously by vortexing for 1 minute.
- Spike-in Addition: Add 5 µL of 5 nM synthetic cel-miR-238 (or cel-miR-39-3p) to monitor extraction efficiency [85] [16].
- Phase Separation:
- Add 1 volume (200 µL) of chloroform.
- Mix vigorously by vortexing for 1 minute.
- Incubate on ice for 2-3 minutes.
- Centrifuge at 12,000 × g at 4°C for 15 minutes.
- Aqueous Phase Transfer: Carefully transfer aqueous phase (approximately 650 µL) to new tube without disturbing interphase [85].
- RNA Precipitation: Add 1.5 volumes (975 µL) of 100% ethanol and mix by pipetting [85].
- Column Purification:
- Elution:
- Centrifuge column at 12,000 × g for 1 minute to remove residual ethanol.
- Transfer column to new collection tube.
- Add 50 µL nuclease-free water to column membrane.
- Incubate at room temperature for 3 minutes.
- Centrifuge at 8,000 × g for 1 minute to elute RNA [85].
- Re-apply eluate to column and repeat centrifugation for higher yield [85].
Quality Control:
- Quantify RNA concentration using fluorometric methods (e.g., Qubit microRNA assay) [16].
- Standardize to 0.25 ng/µL for RT-qPCR analysis [16].
Reverse Transcription and Pre-amplification Protocol
Principle: Stem-loop reverse transcription provides specificity for mature miRNAs. Pre-amplification enables detection of low-abundance miRNAs [85] [16].
Materials:
- TaqMan MicroRNA Reverse Transcription Kit
- Stem-loop RT primers
- dNTPs with dTTP
- RNase inhibitor
- Thermal cycler
Procedure:
- RT Primer Pool Preparation: Prepare multiplex RT primer pool by mixing equal volumes of 20x RT primers for target miRNAs (maximum 4 targets per pool) [85].
- RT Reaction Mix (per reaction):
- 3 µL RT primer pool
- 0.15 µL of 100 mM dNTPs with dTTP
- 1 µL reverse transcriptase (50 U/µL)
- 1.5 µL 10x RT buffer
- 0.19 µL RNase inhibitor (20 U/µL)
- 4.16 µL nuclease-free water
- Total volume: 10 µL [85]
- Reverse Transcription:
- Mix 10 µL RT reaction mix with 5 µL RNA sample.
- Incubate on ice for 5 minutes.
- Run on thermal cycler: 16°C for 30 min, 42°C for 30 min, 85°C for 5 min [85].
- Pre-amplification (for low-abundance miRNAs):
- Perform pre-amplification PCR if Cq values are above 35 [85].
- Use specific pre-amplification primers according to manufacturer's instructions.
Quality Control:
- Include no-template controls (RNase-free water) to monitor contamination [16].
- Use synthetic RNA oligonucleotides for standard curve generation [85].
Quantitative PCR and Data Normalization Protocol
Principle: Accurate quantification requires robust normalization strategies to account for technical variation. Multiple reference normalizers provide superior reliability compared to single reference genes [13].
Materials:
- TaqMan MicroRNA Assays
- qPCR master mix
- qPCR instrument with compatible software
- 96-well or 384-well reaction plates
Procedure:
- Reaction Setup:
- Thermocycling Conditions:
- Initial denaturation: 95°C for 10 min
- 40 cycles of: 94°C for 15 sec, 60°C for 1 min [19]
- Data Analysis:
- Normalization:
Quality Control:
- Assess amplification efficiency (90-110%) from standard curves.
- Monitor inter-plate variation using control samples.
- Maintain consistent analysis software and settings throughout study [13].
Workflow and Signaling Pathway Diagrams
Diagram 1: GLP-Compliant miRNA Analysis Workflow. This workflow outlines the standardized procedure for plasma miRNA analysis, highlighting critical control points and quality assessment steps to ensure reproducibility and reliability.
Research Reagent Solutions
Table 3: Essential Reagents and Materials for GLP-Compliant miRNA Analysis
| Reagent/Material | Function | Specifications | Example Products |
|---|---|---|---|
| K₃EDTA Blood Collection Tubes | Anticoagulant for blood collection | Avoid heparin (PCR inhibitor) | BD Vacutainer K₃EDTA |
| miRNA Extraction Kit | RNA isolation from plasma/serum | Phenol-chloroform method, compatible with small RNAs | miRNeasy Mini Kit |
| Synthetic Spike-in miRNAs | Extraction and RT efficiency control | Non-human origin (e.g., C. elegans) | cel-miR-238, cel-miR-39-3p |
| Stem-loop RT Primers | cDNA synthesis for mature miRNAs | Target-specific reverse transcription | TaqMan MicroRNA Assays |
| qPCR Master Mix | Fluorescent detection of amplified miRNA | Probe-based chemistry recommended | TaqMan Universal PCR Master Mix |
| RNA Quality Assessment Kit | Quantification of extracted miRNA | Fluorometric measurement specific for small RNA | Qubit microRNA Assay Kit |
| Synthetic miRNA Oligos | Standard curve generation | Known concentrations for absolute quantification | Custom RNA oligonucleotides |
| RNase-free Consumables | Prevention of RNA degradation | Low-binding tubes, filter tips | RNase-free microtubes and tips |
The quantification of circulating microRNAs (miRNAs) via reverse transcription quantitative polymerase chain reaction (RT-qPCR) has emerged as a powerful tool in biomedical research, offering significant potential for non-invasive disease diagnosis, prognosis, and therapeutic monitoring [20] [86]. MiRNAs are small, non-coding RNAs approximately 18-24 nucleotides in length that demonstrate remarkable stability in blood plasma despite the presence of ubiquitous RNases [20] [87]. This stability, combined with their disease-specific expression patterns, makes them exceptional candidate biomarkers for a wide spectrum of conditions including cancer, cardiovascular disease, metabolic disorders, and more recently, infectious disease sequelae such as Long COVID [20] [87] [88].
However, the accuracy and reproducibility of miRNA quantification in plasma research is critically dependent on the initial RNA isolation step [89] [86] [90]. The efficiency of miRNA recovery and the purity of the extracted nucleic acids are profoundly influenced by the chosen extraction methodology. Inefficient recovery can lead to false negatives or inaccurate quantification, particularly for low-abundance miRNAs, while impurities co-extracted with RNA can inhibit downstream enzymatic reactions in RT-qPCR, compromising data reliability [86] [90]. This application note systematically compares commercially available RNA extraction methods, evaluating their performance in terms of miRNA recovery efficiency and RNA purity to establish a robust, reproducible protocol for RT-qPCR-based miRNA quantification in plasma.
Key Comparison of RNA Extraction Methods
The selection of an appropriate RNA extraction method is paramount for successful miRNA analysis. The following section provides a detailed comparison of the performance characteristics of several commonly used commercial kits.
Table 1: Comparative Analysis of Commercial Kits for miRNA Isolation from Plasma/Serum
| Kit Name | Principle | Best For | RNA Purity (A260/A280) | miRNA Recovery Efficiency | Key Advantages | Key Limitations |
|---|---|---|---|---|---|---|
| miRNeasy Serum/Plasma Kit (Qiagen) [8] [89] [90] | Phenol/column-based | High miRNA yield from fresh & frozen plasma; Broad miRNA profiling | Acceptable (lower A260/A230 may indicate contaminants) [90] | High; Consistently good detection of a wide range of miRNAs by qPCR [89] [90] | Robust performance across varying input volumes; Less prone to saturation [90] | Potential for contaminant carryover (guanidinium salts, phenol) affecting A260/A230 [90] |
| miRCURY RNA Isolation Kit (Exiqon) [89] [90] | Column-based (phenol-free) | High-purity RNA for sensitive applications | High; Single absorbance peak at 260nm indicates pure RNA [90] | Variable; Efficient from some samples but plateaus with high input due to column saturation [90] | Superior RNA purity; Excellent for downstream applications sensitive to inhibitors [90] | Column capacity can be exceeded with high biomass, limiting miRNA recovery; Performance can vary with sample type [89] [90] |
| Trizol LS Reagent (Invitrogen) [86] [90] | Phenol-based (precipitation) | Maximum flexibility; low-cost option | Low; Significant contaminant carryover (phenol) affects ratios [90] | Lower than column-based methods; Contaminants inhibit RT-qPCR, leading to higher Cq values [90] | Low cost; No specialized equipment needed for basic protocol [86] | Low purity negatively impacts RT-qPCR efficiency and reliability; Requires careful optimization and washing [86] [90] |
| miRNeasy Serum/Plasma Advanced Kit (Qiagen) [20] | Phenol/column-based | Standardized clinical research | Not explicitly stated | High; Validated for stability studies, showing consistent Cq values for key miRNAs [20] | Integrated protocol for biofluids; Used in rigorous biomarker stability studies [20] | - |
Impact of Pre-Analytical Variables and Input Material
Beyond the choice of kit, pre-analytical conditions and the quantity of starting material significantly impact the success of miRNA isolation.
Sample Collection and Storage Stability
Plasma and serum miRNAs demonstrate remarkable stability under various handling conditions. Studies show that miRNA profiles remain consistent even when blood samples are left at room temperature for up to 6 hours before processing, and specific miRNAs (e.g., miR-15b, miR-16, miR-21) show stable Cq values for up to 24 hours when stored on ice [20]. This stability is crucial for accommodating the variability encountered in routine clinical settings.
Quantity of Input Material
The amount of starting plasma directly influences miRNA recovery. A comparative study revealed that kits perform differently across input volumes. For instance, while the miRNeasy and miRCURY kits provided the best miRNA detection from 200 µL of plasma, the performance of some kits significantly declined when used with frozen plasma samples [89]. Furthermore, overloading a kit's column capacity with high biomass can lead to saturation, impeding the binding and recovery of miRNAs, a phenomenon observed with the miRCURY kit [90]. Therefore, using the manufacturer's recommended input volume and ensuring sample consistency within a study is critical.
Assessment of Extraction Efficiency
To control for variations in extraction efficiency between samples, the use of an exogenous spike-in control is strongly recommended. A synthetic, non-mammalian miRNA, such as cel-miR-39, is added to the plasma or serum lysate immediately after sample lysis and prior to the extraction procedure [8] [86] [91]. The recovery of this spike-in control is then measured by RT-qPCR, allowing for normalization of technical variability introduced during the isolation process. Omission of this step can lead to significant misinterpretation of endogenous miRNA levels.
Recommended Protocols
Optimized Protocol for miRNA Isolation from Plasma using miRNeasy Serum/Plasma Kit
This protocol is optimized for balancing high miRNA recovery and compatibility with downstream RT-qPCR [20] [8] [90].
Workflow Overview:
Detailed Steps:
- Plasma Preparation: Collect whole blood in K2EDTA tubes (for plasma) or clotting tubes (for serum). For plasma, centrifuge at 1,200-1,600 × g for 10-15 minutes at room temperature. Transfer the upper plasma layer to a new tube and perform a second centrifugation at 14,400 × g for 10 minutes to remove any remaining cells or debris [20] [8].
- Spike-in Addition: Add a known quantity of synthetic cel-miR-39 (e.g., 1 µL of 1.6 × 10^8 copies/µL) to 100-200 µL of plasma. This controls for extraction efficiency variations [8] [91].
- Lysis and Phase Separation: Add 5 volumes of Qiazol (or TRIzol LS) to the plasma. Vortex thoroughly for 30 seconds. Incubate at room temperature for 5 minutes. Add 1 volume of chloroform (200 µL if using 1 mL Qiazol), vortex vigorously for 30 seconds, and incubate for 2-3 minutes. Centrifuge at 12,000 × g for 15 minutes at 4°C [86] [90].
- RNA Binding: Carefully transfer the upper aqueous phase (approximately 50-60% of the total volume) to a new tube without disturbing the interphase. Add 1.5 volumes of 100% ethanol and mix thoroughly by pipetting. Do not centrifuge. Apply the mixture to the miRNeasy Mini column in successive steps [86].
- Washing: Centrifuge the column and discard the flow-through. Add 700 µL of buffer RWT and centrifuge. Discard the flow-through. Add 500 µL of buffer RPE and centrifuge. Discard the flow-through. Add another 500 µL of buffer RPE and centrifuge for 2 minutes to ensure complete ethanol removal [8].
- Elution: Transfer the column to a new collection tube. Centrifuge at full speed for 1 minute with the lid open to dry the membrane. Elute the RNA by applying 14-30 µL of nuclease-free water directly onto the center of the membrane. Let it stand for 1 minute, then centrifuge at full speed for 1-2 minutes [20] [8].
Protocol for TRIzol LS-based Extraction with Glycogen/Carrier Enhancement
This traditional method requires careful handling to achieve sufficient purity for RT-qPCR [86] [90].
Key Steps:
- Lysis and Phase Separation: Mix 100 µL of plasma with 750 µL of TRIzol LS. Add the cel-miR-39 spike-in. Vortex and add 200 µL of chloroform. Vortex for 30 seconds, incubate for 5 minutes at RT, and centrifuge at 12,000 × g for 15 minutes at 4°C.
- Aqueous Phase Transfer and Precipitation: Transfer 400 µL of the aqueous phase to a new tube. To enhance the precipitation of low-concentration RNA, add 5 µg of glycogen and 100 ng of yeast tRNA as a carrier. Mix well. Add 500 µL of isopropanol, mix by inversion, and incubate at -80°C for at least 1 hour (or overnight for maximum yield).
- Precipitation and Washing: Centrifuge at 20,000 × g for 30 minutes at 4°C to pellet the RNA. Carefully decant the supernatant. Wash the pellet with 700 µL of 70% ethanol by vortexing and centrifuging at 7,500 × g for 5 minutes at 4°C.
- RNA Dissolution: Air-dry the pellet for 5-10 minutes (do not over-dry). Dissolve the RNA in 30 µL of nuclease-free water [86].
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents and Kits for miRNA Extraction and QC
| Item Name | Supplier Examples | Function/Application |
|---|---|---|
| miRNeasy Serum/Plasma Kit | Qiagen | Integrated phenol/column-based system for high-efficiency miRNA isolation from biofluids. |
| miRCURY RNA Isolation Kit - Biofluids | Exiqon (Qiagen) | Phenol-free, column-based system for obtaining high-purity RNA extracts. |
| TRIzol LS Reagent | Invitrogen (Thermo Fisher) | Phenol-based solution for total RNA lysis and extraction; offers protocol flexibility. |
| Streck Cell-Free RNA Blood Collection Tubes | Streck | Stabilizes blood cells and protects cell-free RNA, enabling extended sample transport. |
| Cel-miR-39 Spike-in Control | Qiagen, Thermo Fisher | Synthetic exogenous miRNA added to samples pre-extraction to monitor and normalize for isolation efficiency. |
| Glycogen (Molecular Biology Grade) | Invitrogen, Sigma-Aldrich | Carrier to improve visibility and yield of RNA pellets during precipitation-based methods. |
| Qubit microRNA Assay | Thermo Fisher | Recommended fluorometric method for accurate quantification of low-concentration miRNA extracts. Superior to spectrophotometry for biofluids [89]. |
| TaqMan MicroRNA Reverse Transcription Kit | Applied Biosystems (Thermo Fisher) | For synthesizing cDNA from miRNA templates, often used with specific stem-loop primers for high specificity. |
The choice of RNA extraction method fundamentally influences the success of downstream RT-qPCR analysis of plasma miRNAs. Based on comparative studies, the miRNeasy Serum/Plasma Kit provides a robust balance of high miRNA recovery and operational practicality, making it a strong candidate for routine use in plasma miRNA biomarker research [89] [90]. For applications requiring the highest RNA purity and where input material is not limiting, the miRCURY RNA Isolation Kit is an excellent alternative [90]. The traditional TRIzol LS method, while flexible, requires rigorous optimization and thorough washing to overcome purity issues that compromise RT-qPCR efficacy [86] [90].
To ensure reliable and reproducible results, researchers should:
- Standardize Pre-analytical Conditions: Consistent blood processing and plasma isolation protocols are critical.
- Use a Spike-in Control: Always include an exogenous control like cel-miR-39 to normalize for technical variation in RNA isolation [8] [86].
- Quantify with Fluorometry: Use sensitive methods like the Qubit microRNA assay instead of spectrophotometry for accurate quantification of low-abundance miRNA [89].
- Match Input to Protocol: Adhere to the recommended input volumes for the chosen kit to avoid column saturation or suboptimal yields.
- Validate with Key miRNAs: Include known, stable miRNAs (e.g., miR-16-5p, miR-92a-3p) as endogenous controls in pilot experiments to assess sample quality and RT-qPCR performance [86] [87].
By carefully selecting and optimizing the RNA extraction method within the context of their specific research needs, scientists can lay a solid foundation for accurate and meaningful miRNA quantification in plasma.
Accurate normalization is a critical prerequisite for reliable gene expression analysis using reverse transcription quantitative polymerase chain reaction (RT-qPCR). This is particularly true for the quantification of circulating microRNAs (miRNAs) in plasma, a promising area for minimally invasive biomarker discovery in ageing-related diseases and drug development [13] [71]. The lack of standardized normalization has led to inconsistent data across studies, hindering the clinical implementation of miRNA biomarkers [13]. This Application Note details the experimental protocols and application of three key normalization method validation tools—GeNorm, NormFinder, and BestmiRNorm—within the context of an RT-qPCR workflow for plasma miRNA analysis.
Selecting an appropriate normalization strategy is essential to control for technical variation introduced during sample collection, nucleic acid isolation, and amplification. The following algorithms assist researchers in identifying the most stably expressed reference genes for their specific experimental system.
Comparative Analysis of Normalization Algorithms
The table below summarizes the core characteristics of the three normalization methods.
Table 1: Key Characteristics of GeNorm, NormFinder, and BestmiRNorm
| Method | Core Algorithmic Principle | Primary Output | Optimal Number of Normalizers | Key Strength | Key Limitation |
|---|---|---|---|---|---|
| GeNorm [92] [93] | Pairwise comparison of candidate gene expression ratios to determine the most stable pair. | Expression stability measure (M); lower M value indicates greater stability. Also calculates pairwise variation (V) to determine optimal number of reference genes. | Recommends a pair of genes; the use of more than two is typically unnecessary [93]. | Intuitively identifies the best pair of reference genes. | Does not directly evaluate inter-group variation in cross-sectional studies. |
| NormFinder [93] [94] | Model-based approach that estimates both intra- and inter-group variation. | Stability value; lower value indicates greater stability. | Can recommend a single gene or a pair. | Specifically designed to handle sample subgroups, making it suitable for case-control studies. | More complex computational approach than GeNorm. |
| BestmiRNorm [13] [71] | Novel method utilizing Python to assess a larger number of potential normalizers (up to 11) based on a user-weighted composite score. | A composite score ranking the stability of candidate normalizers. | Flexible; can identify a panel of stable normalizers (e.g., 7 miRNAs) [13]. | High computational efficiency and flexibility for assessing many candidates; transparency in evaluation criteria. | A newer method with less established track record in the literature. |
Experimental Workflow for Validation
The following diagram illustrates the integrated experimental workflow for validating reference genes, from initial sample preparation to final data normalization.
Detailed Experimental Protocols
Sample Preparation and Quality Control
Principle: The integrity of initial plasma samples is paramount. Haemolysis can significantly alter miRNA profiles, as red blood cells are rich in specific miRNAs like miR-451a, leading to normalization artifacts [13].
Protocol:
- Blood Collection: Collect peripheral blood into K₂EDTA tubes [20].
- Plasma Isolation: Centrifuge blood at 1,200–1,500 × g for 10 minutes at room temperature. Carefully transfer the upper plasma layer to a new tube and perform a second centrifugation at 1,500 × g for 5 minutes to remove any residual cells [20] [94].
- Aliquoting and Storage: Aliquot plasma into nuclease-free tubes and store at -80°C until RNA extraction [94].
- Haemolysis Assessment: Evaluate sample quality using one of two methods:
- Absorbance-based method: Measure absorbance at 414 nm (peak for haemoglobin) [13].
- miRNA ratio-based method: Use RT-qPCR to measure ΔCq (Cq miR-23a-3p – Cq miR-451a). A ΔCq < 7 suggests minimal haemolysis, while ΔCq > 7 indicates significant haemolytic contamination [13] [71]. Exclude heavily haemolysed samples.
miRNA Isolation and Reverse Transcription
Principle: Efficient and consistent recovery of miRNA is necessary for accurate quantification. Incorporating spike-in controls monitors technical efficiency.
Protocol:
- miRNA Isolation: Extract total RNA, including miRNAs, from 200-300 µL of plasma using dedicated kits (e.g., miRNeasy Serum/Plasma Kit, Qiagen; or MagMAX miRVana Total Isolation Kit, Thermo Fisher) according to the manufacturer's instructions [20] [68]. Optimization Note: For low-volume paediatric samples, protocol adjustments such as modified elution volumes or reagent ratios may be required with kits like the QIAseq miRNA UDI Library Kit [68].
- Spike-in Controls: Introduce synthetic non-human miRNAs (e.g., C. elegans miR-39-3p) at the start of the isolation process. This controls for variations in RNA extraction and reverse transcription efficiency [94].
- cDNA Synthesis: Synthesize cDNA from a fixed volume of eluted RNA using miRNA-specific stem-loop primers and a High-Capacity RNA-to-cDNA kit [20]. The use of stem-loop primers enhances the specificity and efficiency of reverse transcription for mature miRNAs.
Quantitative PCR (qPCR) Profiling
Principle: To generate high-quality Cq data for candidate reference and target genes with high specificity and efficiency.
Protocol:
- Candidate Gene Selection: Select a panel of candidate reference genes (approximately 7-11) based on literature and preliminary data. For plasma miRNA studies, these are typically endogenous miRNAs previously reported as stable (e.g., miR-103a-2-5p, miR-22-5p, miR-423-5p) [95] [94].
- qPCR Setup: Perform qPCR reactions using TaqMan MicroRNA Assays or SYBR Green chemistry. Run all samples and negative controls in triplicate.
- Data Acquisition: Use a real-time PCR detection system. Critical Note: For a given study, use the same qPCR machine and analysis software for all steps to prevent machine- and software-induced variability [13].
- Quality Checks: Confirm primer specificity via melt-curve analysis (for SYBR Green) and ensure PCR amplification efficiencies are between 90% and 110% [96].
Data Analysis and Validation of Normalizers
Principle: Use algorithmic analysis of Cq values to rank candidate genes by their expression stability.
Protocol:
- Data Input: Compile the Cq values for all candidate reference genes across all samples.
- Algorithmic Analysis:
- GeNorm: Input raw Cq values. The software will calculate an expression stability measure (M) for each gene. Exclude genes with M > 1.0 (default threshold) and identify the top-ranked pair. The pairwise variation (Vn/Vn+1) indicates whether including an additional reference gene is necessary (threshold V < 0.15) [93] [96].
- NormFinder: Input raw Cq values. The algorithm will provide a stability value based on intra- and inter-group variation. Select genes with the lowest stability values [93] [94].
- BestmiRNorm: Input Cq values for up to 11 candidate normalizers. The Python-based script will generate a composite stability score, allowing for the selection of a panel of optimal normalizers [13] [71].
- Consensus Selection: Compare the outputs from all algorithms to identify the most stable reference gene(s) for your experimental conditions.
- Validation: Normalize the expression of a target gene of known behavior using the selected optimal and sub-optimal reference genes. Demonstrate that only the optimal normalizer(s) reveal the expected biological result [92] [97].
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents and Kits for Plasma miRNA RT-qPCR
| Reagent / Kit | Function | Example Product (Vendor) |
|---|---|---|
| RNA Extraction Kit | Isolation of total RNA, including small RNAs, from plasma/serum. | miRNeasy Serum/Plasma Kit (Qiagen), MagMAX miRVana Total Isolation Kit (Thermo Fisher) [20] [68] |
| Spike-in Control miRNA | Synthetic miRNA added to sample lysate to monitor RNA isolation and reverse transcription efficiency. | C. elegans miR-39-3p [94] |
| Reverse Transcription Kit | Synthesis of cDNA from miRNA templates using specific stem-loop primers. | TaqMan Advanced miRNA cDNA Synthesis Kit (Thermo Fisher) [94] |
| qPCR Assays | Sensitive and specific detection of mature miRNAs via qPCR. | TaqMan MicroRNA Assays (Thermo Fisher) [20] [95] |
| qPCR Master Mix | Chemical environment for amplification during qPCR. | iTaq Universal Probes Supermix (Bio-Rad) [20] |
Case Study: Normalization in Alzheimer's Disease Research
A 2023 study optimized an RT-qPCR protocol for circulating miRNA biomarkers in ageing-related diseases like Alzheimer's disease (AD) [13] [71]. The researchers analyzed 140 subjects, including healthy controls and individuals at different AD stages. They assessed 7 candidate endogenous miRNA normalizers using their novel BestmiRNorm method, which was developed to evaluate more candidates than traditional tools. The study identified a combination of 7 stable miRNA normalizers (e.g., miR-103a-2-5p, miR-22-5p) that were consistent across an ageing population, including those with AD [13]. This underscores the importance of rigorous, condition-specific normalization rather than relying on a single, universal reference gene.
Method Selection Guide
The following decision diagram guides the selection of the most appropriate normalization validation method based on key experimental considerations.
The quantification of microRNAs (miRNAs) in plasma has emerged as a promising frontier for developing minimally invasive biomarkers for various diseases, including cancer, neurodegenerative disorders, and ageing-related conditions [20] [13]. These short non-coding RNA molecules, typically 19-25 nucleotides in length, exhibit remarkable stability in circulation and can reflect pathological processes occurring in tissues throughout the body [20] [98]. The accurate measurement of these molecules is therefore critical for both basic research and clinical applications.
Among the available technologies, reverse transcription quantitative PCR (RT-qPCR), microarrays, and digital PCR (dPCR) represent three principal platforms for miRNA profiling, each with distinct technical characteristics, performance metrics, and application suitability [99] [100]. A comprehensive understanding of their relative strengths and limitations enables researchers to select the most appropriate methodology for their specific experimental goals, whether for discovery-phase screening or targeted validation studies. This article systematically evaluates the cross-platform performance of these three technologies within the context of miRNA quantification in plasma, providing detailed application notes and experimental protocols to guide researchers in this rapidly advancing field.
Technology Comparison and Performance Metrics
Technical Characteristics and Performance Parameters
The selection of an appropriate detection platform requires careful consideration of multiple performance parameters relative to experimental objectives. The table below summarizes the key characteristics of RT-qPCR, microarrays, and digital PCR for miRNA analysis in plasma:
Table 1: Cross-Platform Comparison of miRNA Detection Technologies
| Parameter | RT-qPCR | Microarrays | Digital PCR |
|---|---|---|---|
| Quantification Type | Relative (requires standards/controls) | Relative (requires normalization) | Absolute (no standards needed) |
| Throughput | Low to medium (typically < 30 genes) | High (hundreds to thousands) | Medium (limited by partitioning) |
| Dynamic Range | Wide (≥7 logs) [100] | Limited [100] | Wide [54] |
| Sensitivity | High (detects low abundance miRNAs) [100] | Moderate | Very high (detects rare targets & ≤0.1% mutations) [54] |
| Specificity | High (especially with stem-loop designs) [98] | Moderate (challenge with miRNA families) [99] | High [54] |
| Ability to Discover Novel miRNAs | No | No | No |
| Sample Requirement | Low [100] | Moderate | Moderate |
| Tolerance to Inhibitors | Moderate | N/A | High [54] |
| Multiplexing Capability | Low to medium (with optimization) [98] | High (by design) | Low |
| Cost per Sample | Low (for limited targets) | Moderate | High |
| Ease of Data Analysis | Straightforward | Established pipelines [100] | Simple (absolute counts) |
| Best Applications | Targeted validation, small-scale studies [100] | Discovery-phase screening [99] | Absolute quantification, rare target detection [54] |
Performance in miRNA Analysis
When applied specifically to miRNA quantification, each platform faces unique challenges. The short length of mature miRNA sequences constrains probe design for microarrays, often requiring the entire miRNA sequence as a probe, which results in melting temperatures that may vary by more than 20°C [99]. For RT-qPCR, the short sequence length compromises sequence specificity, which is typically addressed through stringent spatial constraints using stem-loop primers [99] [98]. Digital PCR overcomes many quantification challenges through sample partitioning, providing absolute quantification without the need for standard curves [54].
A systematic comparison of miRNA profiling platforms revealed that each technology has distinct advantages depending on the experimental context [99]. Microarrays demonstrated utility for genome-wide screening but with lower dynamic range compared to sequencing-based methods. RT-qPCR provided the sensitivity required for validating candidate miRNAs, while digital PCR offered superior precision for detecting small fold-changes and rare targets [54].
Detailed Experimental Protocols
Optimized RT-qPCR Protocol for Plasma miRNA Quantification
Sample Collection and Quality Control
- Collect peripheral blood using EDTA tubes (for plasma) or clotting tubes (for serum) [20]. For plasma, centrifuge at 1200×g for 10 minutes at room temperature, followed by a second centrifugation at 1500×g for 5 minutes to remove residual cells [20].
- Assess hemolysis using absorbance-based methods (A414/A375 ratios) or the ΔCq method comparing miR-23a-3p (plasma marker) and miR-451a (RBC marker); samples with ΔCq (miR-23a-3p – miR-451a) < 7 are considered acceptable [13].
- Aliquot and store plasma samples at -80°C until RNA extraction; avoid repeated freeze-thaw cycles [17].
miRNA Isolation and Quality Control
- Extract miRNAs using silica column-based methods (e.g., Qiagen miRNeasy Serum/Plasma Kit), which demonstrate superior efficiency compared to TRIzol LS [17].
- Include spike-in controls (e.g., cel-miR-39) at the beginning of extraction to monitor isolation efficiency and account for variations in recovery [13] [17].
- Elute RNA in nuclease-free water (e.g., 28μL) with extended centrifugation time (2 minutes) to maximize yield [20].
Reverse Transcription and qPCR Analysis
- Perform reverse transcription using stem-loop primers specifically designed for mature miRNAs to enhance specificity [98].
- Include both endogenous (e.g., miR-16) and exogenous (e.g., cel-miR-39) controls for normalization to compensate for variations in cDNA synthesis and miRNA recovery [17].
- For qPCR setup, use TaqMan probes or SYBR Green chemistry with the following cycling conditions: initial denaturation at 95°C for 20 seconds, followed by 40 cycles of 95°C for 1 second and 60°C for 20 seconds [3].
- Perform all replicates on the same instrument and analysis software to minimize technical variability [13].
Data Normalization and Analysis
- Implement a robust normalization strategy using multiple stable reference genes. For ageing-related diseases, the following normalizers have demonstrated stability: miR-16-5p, miR-92a-3p, miR-101-3p, miR-125a-5p, miR-146a-5p, miR-222-3p, and let-7g-5p [13].
- Calculate expression levels using the ΔΔCq method, ensuring PCR efficiency falls within acceptable range (90-110%) [13].
The following workflow diagram illustrates the complete RT-qPCR protocol for plasma miRNA analysis:
Microarray Protocol for miRNA Profiling
Sample Preparation and Labeling
- Isolate total RNA including small RNAs using appropriate extraction kits.
- Label miRNAs with fluorescent dyes (e.g., Cy3 or Cy5) using platform-specific labeling kits.
- For dual-color platforms, label test and reference samples with different fluorophores.
Hybridization and Washing
- Hybridize labeled samples to miRNA microarray slides at appropriate temperatures and stringency conditions to minimize cross-hybridization between related miRNA family members [99].
- Wash slides with increasingly stringent buffers to remove non-specifically bound probes.
- For platforms using locked nucleic acid (LNA) probes, optimize hybridization conditions to exploit the higher annealing affinities [99].
Data Acquisition and Analysis
- Scan arrays using appropriate laser settings and photomultiplier tube gains to maximize dynamic range while avoiding saturation.
- Extract fluorescence intensities using feature extraction software.
- Normalize data using global mean normalization or quantile normalization methods [13].
- Identify differentially expressed miRNAs using appropriate statistical methods with multiple testing correction.
Digital PCR Protocol for Absolute miRNA Quantification
Sample Preparation and Partitioning
- Prepare PCR reaction mix containing TaqMan assays, master mix, and template cDNA.
- Partition samples into thousands of individual reactions using either droplet-based or nanoplate-based systems [54].
- For nanoplate-based dPCR, transfer master mix, probes, primers, and samples to a 96-well nanoplate [54].
Amplification and Reading
- Amplify partitions using endpoint PCR cycling conditions.
- Read partitions simultaneously using integrated imaging systems [54].
- Analyze fluorescence in each partition to determine the ratio of positive to negative reactions.
Data Analysis and Absolute Quantification
- Apply Poisson statistical analysis to determine the absolute copy number of the target miRNA in the original sample [54].
- Calculate concentration in copies per microliter without the need for standard curves [54].
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagent Solutions for Circulating miRNA Analysis
| Reagent/Category | Specific Examples | Function & Importance |
|---|---|---|
| RNA Isolation Kits | miRNeasy Serum/Plasma Kit (Qiagen) | Specialized for low-abundance miRNA from biofluids; includes QC steps for consistent yield [20] [3]. |
| Spike-in Controls | cel-miR-39, syn-cel-miR-39, UniSp2/3/4 | Normalization across extraction and RT; corrects for technical variation [13] [17]. |
| Stem-loop RT Primers | TaqMan Advanced miRNA cDNA Synthesis Kit | Increases specificity for mature miRNAs by creating extended cDNA templates [3] [98]. |
| qPCR Master Mixes | TaqMan Fast Advanced Master Mix, iTaq Universal Probes Supermix | Provides optimized enzyme systems for efficient amplification of miRNA targets [20] [3]. |
| Reference Genes | miR-16-5p, miR-92a-3p, let-7g-5p (plasma-specific) | Stable endogenous normalizers identified for ageing populations and disease states [13]. |
| Hemolysis Detection | miR-23a-3p/miR-451a assay, spectrophotometric (A414/A375) | Quality control; excludes samples with RBC contamination that alters miRNA profiles [13]. |
| dPCR Reagents | QIAcuity nanoplate kits, droplet digital PCR supermixes | Enables absolute quantification without standard curves; ideal for low-abundance targets [54]. |
Application Case Studies
miRNA Biomarkers in Alzheimer's Disease
An optimized RT-qPCR approach was developed for validating circulating miRNA biomarkers in ageing-related diseases such as Alzheimer's disease [13]. The protocol incorporated absorbance-based haemolysis detection, double spike-in controls for miRNA isolation and reverse transcription, and seven stable normalizers verified in 140 subjects. The study highlighted the critical importance of consistent instrumentation and analysis software, as different platforms (StepOnePlus and 7900HT) and software packages (Sequence Detection System v. 2.4 and ExpressionSuite Software v1.3) introduced significant variability in Cq values, potentially impacting results interpretation [13].
Medication-Related Osteonecrosis of the Jaw
A comprehensive profiling study compared plasma and exosomal miRNAs in patients with medication-related osteonecrosis of the jaw (MRONJ) [3]. The research employed a three-phase approach: initial screening using qPCR array plates targeting 754 miRNAs, validation with individual qPCR assays across the full cohort, and confirmation in plasma-derived exosomes. The study identified four miRNAs (miR-483-5p, miR-92-5p, miR-628-3p, and miR-486-5p) as potential biomarkers, demonstrating consistent expression patterns in both plasma and exosomal fractions [3].
Ovarian Germ Cell Tumors
A recent study matched plasma and tissue miRNA expression to detect viable ovarian germ cell tumors [101]. Researchers measured miR-371-3 and miR-302/367 levels using RT-qPCR in 23 patients with ovarian germ cell tumors. The results showed that compared to healthy controls, all patients with viable non-teratoma germ cell tumors had significantly higher miRNA levels in preoperative plasma and tumor tissue. Plasma miRNA kinetics correlated with disease burden, decreasing to undetectable levels following treatment and increasing significantly upon relapse, demonstrating the utility of miRNA quantification for disease monitoring [101].
Technology Selection Framework
The following decision diagram guides researchers in selecting the most appropriate platform for their specific experimental needs:
The selection of an appropriate analytical platform for miRNA quantification in plasma depends primarily on the experimental objectives, with each technology offering distinct advantages. RT-qPCR remains the gold standard for targeted validation studies, providing an optimal balance of sensitivity, specificity, and cost-effectiveness for focused investigations. Microarrays offer a robust solution for discovery-phase screening of multiple miRNA targets simultaneously, while digital PCR provides superior precision for absolute quantification of low-abundance targets and detection of small fold-changes.
Critical to success across all platforms is the implementation of rigorous quality control measures, including proper sample handling, hemolysis assessment, use of spike-in controls, and application of appropriate normalization strategies. The continued refinement of these methodologies will undoubtedly enhance the reliability of circulating miRNA measurements and accelerate their translation into clinically useful biomarkers for human diseases.
Inter-laboratory Reproducibility and Standardization Initiatives
The quantification of circulating microRNAs (miRNAs) in plasma holds immense promise as a non-invasive approach for biomarker discovery and clinical diagnostics. However, the translation of these findings into clinically applicable tools has been hampered by a critical challenge: a lack of inter-laboratory reproducibility. Inconsistent results across different research centers undermine the reliability of data, preventing the validation of miRNA biomarkers and their adoption in clinical practice. This application note examines the primary sources of this variability and outlines established and emerging standardization initiatives designed to overcome these hurdles, with a specific focus on RT-qPCR-based miRNA quantification in plasma.
The MIQE (Minimum Information for Publication of Quantitative Real-Time PCR Experiments) guidelines represent a cornerstone effort to address the reproducibility crisis in qPCR. The recent release of MIQE 2.0 underscores the ongoing need for methodological rigor, emphasizing that "without methodological rigour, data cannot be trusted" [102]. These guidelines provide a comprehensive framework for transparency in qPCR experiments, covering sample handling, assay validation, and data analysis. Despite widespread awareness of MIQE, compliance remains problematic, and the technique is often treated as a "black box," leading to fundamental methodological failures that compromise data integrity [102].
The journey from blood draw to a quantitative miRNA result is fraught with potential sources of variation. These can be categorized into pre-analytical, analytical, and post-analytical factors.
Pre-Analytical Variables
Pre-analytical variables, pertaining to sample collection and processing, constitute a significant source of irreproducibility.
- Sample Matrix Selection: The choice between plasma and serum can systematically influence miRNA measurements. Studies have demonstrated that serum generally provides higher miRNA yields than plasma. For instance, in feline models, serum yielded significantly higher levels of miR-20a (p < 0.0001) and miR-16-5p (p < 0.0002) compared to plasma [16]. Serum preparation, however, carries a higher risk of enriching miRNAs from platelets and red blood cells (RBCs) due to cell lysis during clot formation [103].
- Hemolysis: Hemolysis is a critical confounder, as it leads to the release of cellular miRNAs, notably from RBCs. miRNAs like miR-16 and miR-451a are highly abundant in RBCs, and their levels in plasma can be drastically elevated in hemolyzed samples, skewing profiles and compromising the accuracy of endogenous normalizers [103] [57].
- Sample Processing and Storage: Variables such as the time interval between blood collection and processing, centrifugation speed and duration, temperature during handling, and the number of freeze-thaw cycles can alter miRNA profiles [103] [20]. While miRNAs demonstrate remarkable stability—with one study showing over 99% of the miRNA profile unchanged after blood tubes were left at room temperature for 6 hours [20]—standardizing these steps remains crucial for comparability.
Table 1: Key Pre-analytical Confounding Factors and Their Impact
| Confounding Factor | Impact on miRNA Quantification |
|---|---|
| Hemolysis | Releases RBC-specific miRNAs (e.g., miR-16, miR-451a), altering the true plasma miRNA profile [103] [57]. |
| Sample Type (Plasma vs. Serum) | Systematic differences in yield; serum can be enriched with miRNAs from platelets and RBCs during clotting [103] [16]. |
| Time to Processing | Can affect miRNA stability, though miRNAs are generally stable over short periods [20]. |
| Freeze-Thaw Cycles | Repeated cycles can degrade miRNA and reduce available molecule numbers [103]. |
| Anticoagulant Use | The type of anticoagulant (e.g., EDTA, heparin) can influence downstream RT-qPCR efficiency [57]. |
Analytical Variables
Analytical variability arises during the experimental workflow of miRNA isolation, reverse transcription, and qPCR.
- miRNA Isolation: The choice of isolation methodology (e.g., phenol-based vs. column-based) and the use of RNA carriers (e.g., yeast RNA, MS2 RNA) significantly impact the efficiency and bias of miRNA recovery. The addition of a carrier like yeast RNA generally increases recovery, but this improvement is dependent on the GC content and thermodynamic stability of the specific miRNA, potentially introducing compositional bias [104].
- Data Normalization: This is arguably the most critical analytical step for achieving reproducible data. Normalization controls for technical variations in RNA input, isolation efficiency, and enzymatic reactions. The use of inappropriate reference genes is a widespread failure [102]. A common but suboptimal strategy is using a single, presumed stable endogenous miRNA (e.g., miR-16 or miR-191) without prior validation. miR-16, for instance, is not a universal normalizer as its expression can vary with hemolysis and specific disease states like COVID-19 [25] [57]. Best practice involves the use of a combination of stable normalizers identified for the specific experimental context. For example, a study on ageing and Alzheimer's disease validated a set of 7 stable normalizers for plasma miRNA analysis in these populations [13]. The use of exogenous spike-in controls (e.g., cel-miR-39) is highly recommended to monitor isolation and reverse transcription efficiency, though they are best used for quality control rather than as the sole normalization factor [25] [26].
Table 2: Advantages and Disadvantages of Common Normalization Strategies
| Normalization Strategy | Advantages | Disadvantages |
|---|---|---|
| Endogenous miRNAs (e.g., miR-16-5p) | Corrects for technical variations; biologically relevant. | Must be validated for each specific condition; stability can be affected by hemolysis and pathology [25] [57]. |
| Exogenous Spike-in (e.g., cel-miR-39) | Monitors technical efficiency of isolation and RT; added after sample collection. | Does not control for biological variation; amount added must be optimized and consistent [25] [26]. |
| Global Mean Normalization | Robust for high-throughput datasets (e.g., RNA-Seq). | Less reliable for low-target-number experiments like RT-qPCR [13]. |
| Combination of Validated Normalizers | Most robust approach; reduces variance. | Requires upfront validation using algorithms like NormFinder or BestmiRNorm [13]. |
Standardization Initiatives and Best-Practice Protocols
Adherence to MIQE 2.0 Guidelines
The MIQE 2.0 guidelines provide an authoritative and detailed framework to remedy deficiencies in qPCR experiments [102]. For inter-laboratory reproducibility, adherence to these guidelines is non-negotiable. Key aspects include:
- Detailed Sample Description: Reporting of pre-analytical variables such as sample matrix, collection tube, processing delay, and storage conditions.
- Assay Validation: Providing data on primer sequences, PCR efficiencies, and dynamic range for each assay.
- qPCR Data Transparency: Reporting of raw Cq values, normalization methods, and the number of technical replicates.
- Statistical Justification: Clear description of statistical methods and the number of biological replicates.
A Standardized Workflow for Plasma miRNA Quantification by RT-qPCR
The following protocol synthesizes best practices from the literature to enhance reproducibility.
Advanced Normalization and Quality Control
To address the critical challenge of normalization, a novel method called BestmiRNorm has been developed. This computational tool, implemented in Python, allows for the assessment of a larger number of potential normalizers (up to 11) compared to existing algorithms like NormFinder and GeNorm. It provides a clear evaluation basis and allows researchers to weight the evaluation according to the relative importance of different features, facilitating the identification of an optimal set of normalizers for specific experimental conditions, such as in ageing-related diseases [13].
Quality control for hemolysis should employ a dual-method approach:
- Absorbance-based detection: Measure absorbance at 414 nm for hemoglobin.
- miRNA ratio-based detection: Use the ΔCq between the RBC-enriched miR-451a and a plasma marker like miR-23a-3p. A ΔCq (miR-23a-3p – miR-451a) < 7 is a commonly used threshold to indicate minimal hemolysis [13].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagent Solutions for Plasma miRNA Analysis
| Item | Function | Example & Note |
|---|---|---|
| K₂EDTA Blood Collection Tubes | Plasma separation; inhibits coagulation. | Preferred over heparin (a PCR inhibitor) for molecular work [57]. |
| Column-based miRNA Isolation Kit | Purification of small RNAs from biofluids. | Qiagen miRNeasy Serum/Plasma kit; often used with RNA carriers [104] [13]. |
| RNA Carrier (e.g., Yeast RNA) | Improves miRNA recovery during isolation. | Increases yield, particularly for miRNAs with low GC content; masks spectrophotometric quantification [104]. |
| Exogenous Spike-in Control (cel-miR-39) | Monitors technical efficiency of isolation and RT. | Added to lysis buffer; not a standalone normalizer [25] [26]. |
| Stem-loop RT Primers & TaqMan Assays | Specific reverse transcription and amplification of mature miRNAs. | Applied Biosystems TaqMan MicroRNA Assays; gold standard for specificity [16] [13]. |
| Validated Endogenous Normalizers | Stable reference for data normalization. | Must be context-specific; e.g., a panel of 7 miRNAs was identified for ageing/AD studies [13]. |
Achieving inter-laboratory reproducibility in plasma miRNA quantification is a complex but attainable goal. It requires a concerted shift from treating RT-qPCR as a simple, off-the-shelf technique to implementing a culture of rigorous methodology and transparency. The path forward hinges on the widespread adoption of standardized protocols that meticulously control for pre-analytical and analytical variables, coupled with strict adherence to the MIQE 2.0 guidelines. The implementation of robust, context-specific normalization strategies, validated using tools like BestmiRNorm, is paramount. By embracing these initiatives, the research community can overcome the current reproducibility crisis and unlock the full potential of circulating miRNAs as reliable biomarkers for disease diagnosis and drug development.
Conclusion
Standardized RT-qPCR protocols for plasma miRNA quantification are essential for translating promising biomarker research into clinically applicable tools. This comprehensive workflow integrates critical advancements in pre-analytical processing, robust normalization using spike-in controls like cel-miR-39, systematic quality control for hemolysis and inhibitors, and rigorous validation following good laboratory practices. By addressing key sources of variability and implementing the optimization strategies outlined, researchers can significantly improve the reproducibility and reliability of their miRNA data. Future directions should focus on establishing universally accepted reference materials, developing automated high-throughput workflows, and validating disease-specific miRNA panels for early diagnosis, patient stratification, and treatment monitoring in precision medicine applications.
References
- 1. and High-Dose Whole-Body Ionizing Radiation Exposure [https://pmc.ncbi.nlm.nih.gov/articles/PMC12559630/]
- 2. Analysis of the longitudinal stability of human plasma ... [https://www.nature.com/articles/s41598-024-52681-5]
- 3. Comprehensive profiling of plasma and exosomal ... [https://www.nature.com/articles/s41598-025-21269-y]
- 4. Stability of microRNAs in serum and plasma reveal ... [https://pubmed.ncbi.nlm.nih.gov/40926836/]
- 5. Stability of microRNAs in serum and plasma reveal ... [https://www.sciencedirect.com/science/article/pii/S2468054025000897]
- 6. JoVE | Peer Reviewed Scientific Video Journal - Methods and Protocols [https://www.jove.com/t/56850/-microrna-pcr]
- 7. Droplet digital polymerase -based chain of... reaction quantification [https://www.nature.com/articles/s41598-020-66072-z?error=cookies_not_supported&code=45199550-1653-4883-ad90-e03c2405d056]
- 8. Matching plasma and tissue miRNA expression analysis to ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0322477]
- 9. A comparison of RNA extraction and sequencing protocols for... [https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-019-5826-7]
- 10. The emerging role of miRNAs in biological aging and age- ... [https://pubmed.ncbi.nlm.nih.gov/40501482/]
- 11. MicroRNAs as Peripheral Biomarkers in Aging and Age- ... [https://ohsu.elsevierpure.com/en/publications/micrornas-as-peripheral-biomarkers-in-aging-and-age-related-disea]
- 12. Plasma microRNA signatures of aging and their links to health ... [https://genomemedicine.biomedcentral.com/articles/10.1186/s13073-025-01437-5]
- 13. Optimized RT-qPCR and a novel normalization method for ... [https://www.nature.com/articles/s41598-023-47971-3]
- 14. Cancer and Aging Biomarkers: Classification, Early ... [https://www.mdpi.com/2079-6374/15/11/737]
- 15. Common diseases alter the physiological age-related ... [https://www.nature.com/articles/s41467-020-19665-1]
- 16. RT-rtPCR quantification of circulating microRNAs in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8546485/]
- 17. Quantification of Circulating miRNAs in Plasma: Effect ... [https://www.sciencedirect.com/science/article/pii/S1525157813001372]
- 18. Plasma microrna quantification protocol - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10914336/]
- 19. Quantification of plasma miRNA levels using reverse ... [https://bio-protocol.org/exchange/minidetail?id=8403651&type=30]
- 20. Stability of microRNAs in serum and plasma reveal promise as ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12414832/]
- 21. Protocols for the analysis of microRNA expression ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6727972/]
- 22. Advancing quantitative PCR with color cycle multiplex ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11417387/]
- 23. Preanalytical, analytical and postanalytical considerations ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11177657/]
- 24. Impact of preanalytical and analytical conditions | PLOS One [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0201069]
- 25. Data normalization of plasma miRNA profiling from patients ... [https://www.nature.com/articles/s41598-024-75740-3]
- 26. Plasma microrna quantification protocol [https://www.oaepublish.com/articles/2574-1209.2023.69]
- 27. Brief guide to RT-qPCR - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11612376/]
- 28. Optimization Tips for Luna® One-Step RT-qPCR [https://www.neb.com/en-us/tools-and-resources/usage-guidelines/optimization-tips-for-luna-one-step-rt-qpcr?srsltid=AfmBOopNsSDlRhnGy1_isSPa0iY2FTXtnQ-U0yBExH9NR_gdw_S-vKnX]
- 29. Consensus guidelines for the validation of qRT-PCR ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8792405/]
- 30. Quantitative PCR validation for research scientists: Part 1 of 2 [https://virologyresearchservices.com/2023/01/13/quantitative-pcr-validation-part-1-of-2/]
- 31. qPCR and RT-qPCR [https://www.promega.com/resources/guides/nucleic-acid-analysis/qpcr-and-rt-qpcr-amplification/]
- 32. Validation of current practice and a near patient testing ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC1296733/]
- 33. Comparison of Modified Manual Acid-Phenol Chloroform ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10275685/]
- 34. Improved protocol for plasma microRNA extraction and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8495618/]
- 35. The top advantages and disadvantages of different RNA ... [https://rochesequencingstore.com/resources/the-top-advantages-and-disadvantages-of-different-rna-extraction-methods/]
- 36. Phenol-Chloroform Extraction - an overview [https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/phenol-chloroform-extraction]
- 37. Total RNA Purification Kits [https://norgenbiotek.com/product/total-rna-purification-kits?srsltid=AfmBOooZO2mShxR-9sHuIyUbnZLidkfBq0aNIYs1J2Zeq7xlZi_vyoJP]
- 38. microRNA (cel-miR-39) Spike-In Kit [https://norgenbiotek.com/product/microrna-cel-mir-39-spike-kit?srsltid=AfmBOor4ZlWyi2UnrTSTnmVXwUPMtC1rdKPEU4zf2w7qS7fWK8sU6oGG]
- 39. Evaluation of several methodological challenges in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5898892/]
- 40. - Stem RT- loop for miRNAs qPCR [https://pubmed.ncbi.nlm.nih.gov/21732315/]
- 41. RT- qPCR with chimeric dU stem - loop is efficient for the... primer [https://link.springer.com/article/10.1007/s00253-017-8181-0]
- 42. rtpcr: a package for statistical analysis and graphical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12530193/]
- 43. How To Interpret RT-qPCR Results [https://www.goldbio.com/blogs/articles/how-to-interpret-rt-qpcr-results?srsltid=AfmBOoqWVnNLuYYFkhf5pa9whS0la3WjGmBKDH7min-JJkza57KfcVS9]
- 44. Rapid and accurate quantification of isomiRs by RT-qPCR [https://www.nature.com/articles/s41598-022-22298-7]
- 45. Protocol: a highly sensitive RT-PCR method for detection and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2225395/]
- 46. Quantification of miRNAs by RT-qPCR [https://bio-protocol.org/exchange/minidetail?id=2591265&type=30]
- 47. Optimization Tips for Luna® One-Step RT-qPCR [https://www.neb.com/en-us/tools-and-resources/usage-guidelines/optimization-tips-for-luna-one-step-rt-qpcr?srsltid=AfmBOopwinKNFNtSlm7KE2FklX8aOCqVNboMxhcFHRX5N7seHnr0twTW]
- 48. Basic Principles of RT-qPCR [https://www.thermofisher.com/us/en/home/brands/thermo-scientific/molecular-biology/molecular-biology-learning-center/molecular-biology-resource-library/spotlight-articles/basic-principles-rt-qpcr.html]
- 49. How To Interpret RT-qPCR Results [https://www.goldbio.com/blogs/articles/how-to-interpret-rt-qpcr-results?srsltid=AfmBOoqHFxHEcfiJN-O_ZPaTS-zveESCYYBK8ntaZyMixVwDaiDYrYzc]
- 50. An optimized protocol for stepwise optimization of real-time ... [https://www.nature.com/articles/s41438-021-00616-w]
- 51. Extraction-Free Absolute Quantification of Circulating ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9220272/]
- 52. A comparison between quantitative PCR and droplet digital ... [https://bmcbiotechnol.biomedcentral.com/articles/10.1186/s12896-016-0292-7]
- 53. Development of an innovative duplex digital PCR assay for ... [https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-025-06941-1]
- 54. dPCR vs qPCR [https://www.qiagen.com/us/applications/digital-pcr/beginners/dpcr-vs-qpcr]
- 55. A robust ddPCR assay for the absolute quantification of ... [https://pubmed.ncbi.nlm.nih.gov/41167182/]
- 56. Multiplex digital PCR for the simultaneous quantification of ... [https://www.sciencedirect.com/science/article/pii/S0003267024012418]
- 57. Considerations and Suggestions for the Reliable Analysis ... [https://www.mdpi.com/2073-4425/13/2/328]
- 58. Comparison of Methodologies to Detect Low Levels ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4824492/]
- 59. Haemolysis Detection in MicroRNA-Seq from Clinical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9317737/]
- 60. Haemolysis Detection in MicroRNA-Seq from Clinical ... [https://www.mdpi.com/2073-4425/13/7/1288]
- 61. Considerations and Suggestions for the Reliable Analysis of miRNA ... [https://ncbi.nlm.nih.gov/pmc/articles/PMC8872398]
- 62. Two-tailed RT-qPCR panel for quality control of circulating microRNA studies | Scientific Reports [https://www.nature.com/articles/s41598-019-40513-w]
- 63. Assessment of PCR Inhibitor Removal Methods to Monitor ... [https://link.springer.com/article/10.1007/s11270-023-06821-8]
- 64. A rapid and high-yield method for nucleic acid extraction [https://www.nature.com/articles/s41598-025-95226-0]
- 65. Comparison evaluation of bacterial DNA extraction ... [https://www.nature.com/articles/s41598-025-87225-y]
- 66. An optimized protocol for stepwise optimization of real-time RT ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8325682/]
- 67. Understanding qPCR Efficiency and Why It Exceeds 100% [https://biosistemika.com/blog/qpcr-efficiency-over-100/]
- 68. miRNA Library Preparation Optimisation for Low ... [https://www.mdpi.com/2311-553X/11/1/11]
- 69. Optimization Tips for Luna® One-Step RT-qPCR [https://www.neb.com/en-us/tools-and-resources/usage-guidelines/optimization-tips-for-luna-one-step-rt-qpcr?srsltid=AfmBOoqlmfzLHs2VMeiKERCee1i7IYmdoToNFdd9mIJ1x7SlCgjI8K0R]
- 70. microRNA & Noncoding RNA Analysis Using Real-Time PCR [https://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/real-time-pcr-applications/microrna-noncoding-rna-with-real-time-pcr.html]
- 71. Optimized RT-qPCR and a novel normalization method ... - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10682428/]
- 72. Identification of robust RT-qPCR reference genes for studying ... [https://bmcgenomics.biomedcentral.com/articles/10.1186/s12864-025-11216-6]
- 73. A comparison of normalization methods for the expression of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12312509/]
- 74. Emerging frontiers in microRNA technology: Innovations ... [https://www.sciencedirect.com/science/article/abs/pii/S0142961225006350]
- 75. Digital PCR Breaks Into the Mainstream in 2025 - Vocal Media [https://vocal.media/feast/digital-pcr-breaks-into-the-mainstream-in-2025]
- 76. The Impact of the Variability of RT-qPCR Standard Curves ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12029521/]
- 77. A Novel High-Fidelity DNA Polymerase-driven or Taq ... [https://www.sciencedirect.com/science/article/abs/pii/S0925400525013358]
- 78. Improved protocol for plasma microRNA extraction and ... [https://www.semanticscholar.org/paper/Improved-protocol-for-plasma-microRNA-extraction-of-Sriram-Khanka/74dc00e0154b7d4fc8e97e5fbbea6a41007bf55d]
- 79. Real-Time PCR Basics Support—Troubleshooting [https://www.thermofisher.com/us/en/home/technical-resources/technical-reference-library/real-time-digital-PCR-applications-support-center/real-time-pcr-basics-support/real-time-pcr-basics-support-troubleshooting.html]
- 80. qPCR Troubleshooting: How to Ensure Successful ... [https://dispendix.com/blog/qpcr-troubleshooting]
- 81. Troubleshooting qPCR: Interpreting Amplification Curves [https://blog.biosearchtech.com/thebiosearchtechblog/bid/63622/qpcr-troubleshooting]
- 82. Troubleshooting fine‐tuning procedures for qPCR system ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6647679/]
- 83. Nanomaterial-driven hypersensitive microRNA detection ... [https://www.sciencedirect.com/science/article/pii/S1385894725101976]
- 84. miRNAs: The Road from Bench to Bedside - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC9957002/]
- 85. Absolute Quantification of Plasma MicroRNA Levels in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5912405/]
- 86. Screening of miRNAs in plasma as a diagnostic biomarker for ... [https://bmcbiotechnol.biomedcentral.com/articles/10.1186/s12896-021-00710-w]
- 87. MicroRNAs in long COVID: roles, diagnostic biomarker ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12352008/]
- 88. A preliminary assessment of a stool-based microRNA ... [https://www.nature.com/articles/s41598-025-14485-z]
- 89. Comparison of methods for miRNA isolation and ... [https://www.nature.com/articles/s41598-020-57659-7]
- 90. Assessing cellular and circulating miRNA recovery [https://www.nature.com/articles/srep19529]
- 91. Matching plasma and tissue miRNA expression analysis to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12063854/]
- 92. of reference genes for gene expression analysis in chicory... Validation [https://pubmed.ncbi.nlm.nih.gov/20156357/]
- 93. of endogenous reference genes for qRT-PCR analysis of... Validation [https://bmcmolbiol.biomedcentral.com/articles/10.1186/1471-2199-11-39]
- 94. Identification and validation of endogenous control ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7719248/]
- 95. Identification of suitable plasma-based reference genes for ... [https://pubmed.ncbi.nlm.nih.gov/24479999/]
- 96. Selection of reference genes for gene expression analysis ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7925589/]
- 97. Identification and Validation of Quantitative PCR Reference Genes... [https://currents.plos.org/md/article/identification-and-validation-of-quantitative-pcr-reference-genes-suitable-for-normalizing-expression-in-normal-and-dystrophic-cell-culture-models-of-myogenesis/]
- 98. An ultrasensitive and multiplexed miRNA one-step real ... [https://www.sciencedirect.com/science/article/abs/pii/S0956566323008692]
- 99. Systematic comparison of microarray profiling, real-time ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC2856892/]
- 100. qPCR, Microarrays or RNA Sequencing - What to Choose? [https://biosistemika.com/blog/qpcr-microarrays-rna-sequencing-choose-one/]
- 101. Matching plasma and tissue miRNA expression analysis to ... [https://pubmed.ncbi.nlm.nih.gov/40343905/]
- 102. MIQE 2.0 and the Urgent Need to Rethink qPCR Standards [https://www.mdpi.com/1422-0067/26/11/4975]
- 103. Pre-analytical considerations for microRNA quantification ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12342214/]
- 104. Comparison of protocols and RNA carriers for plasma miRNA ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0187005]