Liquid Biopsy Workflow for Cancer Monitoring: A Comprehensive Guide for Precision Oncology and Drug Development
This article provides a comprehensive overview of the liquid biopsy workflow for cancer monitoring, tailored for researchers and drug development professionals. It explores the foundational principles of circulating biomarkers, including ctDNA, CTCs, and epigenetic markers. The content details advanced methodological approaches from sample collection to data analysis, addresses key challenges in sensitivity and standardization, and presents recent validation studies and comparative performance data. By synthesizing current evidence and future directions, this guide aims to support the integration of liquid biopsy into clinical trial design and precision oncology strategies, enabling non-invasive, real-time tumor dynamics monitoring.
Liquid Biopsy Workflow for Cancer Monitoring: A Comprehensive Guide for Precision Oncology and Drug Development
Abstract
This article provides a comprehensive overview of the liquid biopsy workflow for cancer monitoring, tailored for researchers and drug development professionals. It explores the foundational principles of circulating biomarkers, including ctDNA, CTCs, and epigenetic markers. The content details advanced methodological approaches from sample collection to data analysis, addresses key challenges in sensitivity and standardization, and presents recent validation studies and comparative performance data. By synthesizing current evidence and future directions, this guide aims to support the integration of liquid biopsy into clinical trial design and precision oncology strategies, enabling non-invasive, real-time tumor dynamics monitoring.
The Foundation of Liquid Biopsy: Core Biomarkers and Biological Principles for Cancer Monitoring
Circulating Tumor DNA (ctDNA)
Circulating tumor DNA (ctDNA) refers to small fragments of tumor-derived DNA found in biofluids such as blood, carrying tumor-specific genomic alterations. It represents a dynamic snapshot of tumor burden and heterogeneity, with a short half-life of approximately 16 minutes to several hours, enabling real-time monitoring of disease [1].
Table 1: Key Analytical Technologies for ctDNA Detection
| Technology | Key Feature | Reported Sensitivity | Primary Application |
|---|---|---|---|
| Structural Variant (SV) Assays | Detects tumor-specific rearrangements; avoids PCR errors [2]. | Parts-per-million (e.g., VAF < 0.01%) [2] | MRD, early recurrence detection |
| Nanomaterial-based Electrochemical Sensors | Uses graphene/MoSâ‚‚ for label-free impedance sensing; rapid results [2]. | Attomolar (within 20 mins) [2] | Point-of-care diagnostics |
| PhasED-Seq | Targets multiple SNVs on the same DNA fragment [2]. | High sensitivity for low VAF [2] | MRD detection |
| Fragmentomics & Size Selection | Enriches shorter ctDNA fragments (90-150 bp) vs. longer non-tumor cfDNA [2]. | Several-fold increase in abundance [2] | Enhances all NGS-based assays |
| Error-Corrected NGS (e.g., CAPP-Seq, TEC-Seq) | Uses UMIs and duplex sequencing to filter sequencing artifacts [1]. | ~0.01% VAF [1] | Profiling, therapy selection, MRD |
Protocol 1: ctDNA Analysis for Treatment Response Monitoring
- Sample Collection: Collect 10-20 mL of peripheral blood into cell-free DNA blood collection tubes (e.g., Streck, PAXgene). Centrifuge within 2-6 hours to separate plasma, followed by a second high-speed centrifugation to remove residual cells [2] [1].
- cfDNA Extraction: Isolate cfDNA from plasma using commercial silica-membrane or magnetic bead-based kits (e.g., QIAamp Circulating Nucleic Acid Kit). Quantify yield using fluorometry [1].
- Library Preparation & Sequencing:
- For tumor-informed MRD: Perform whole-genome sequencing (WGS) on tumor tissue to identify patient-specific somatic variants (SNVs, SVs). Design a custom hybrid-capture panel or multiplex PCR assay targeting these variants [2] [1].
- For tumor-agnostic profiling: Use a targeted NGS panel for common cancer driver genes (e.g., KRAS, EGFR, PIK3CA).
- Incorporate Unique Molecular Identifiers (UMIs) during library construction to enable error correction [1].
- For increased sensitivity, perform biomechanical or enzymatic size selection to enrich for shorter DNA fragments [2].
- Bioinformatic Analysis: Process sequencing data with a pipeline that includes UMI consensus building, alignment, variant calling, and error suppression (e.g., using AI-based methods) to distinguish true low-frequency variants from noise [2] [1].
Circulating Tumor Cells (CTCs)
Circulating Tumor Cells (CTCs) are intact, rare cells shed from primary or metastatic tumors into the bloodstream. Their analysis provides insights into metastatic potential, allowing for functional characterization and protein expression analysis [3] [4].
Table 2: Key Analytical Technologies for CTC Isolation and Analysis
| Technology/Platform | Principle | Key Application | Clinical Utility |
|---|---|---|---|
| CellSearch | FDA-cleared; immunomagnetic enrichment (EpCAM+) [3]. | Prognosis in metastatic breast, prostate, CRC [3]. | Established prognostic biomarker |
| Microfluidic Labyrinth | Antigen-independent; exploits cell size/deformability [4]. | Enumeration & scRNA-seq in DCIS [4]. | Investigational; risk stratification |
| AR-V7 Testing | Detects androgen receptor splice variant in CTCs [3]. | Therapy selection in mCRPC [3]. | Predictive biomarker |
| scRNA-seq | Molecular profiling of single CTCs [4]. | Clonal evolution, EMT assessment [4]. | Research & biomarker discovery |
Protocol 2: CTC Enrichment and Single-Cell RNA Sequencing
- Sample Collection & Shipping: Draw 20-30 mL of peripheral blood into specialized preservative tubes (e.g., CellSave for enumeration, CellRescue for functional analysis). For multi-site studies, ship overnight at ambient temperature [4].
- CTC Enrichment (via Labyrinth):
- Process blood samples through the microfluidic Labyrinth device. This device uses inertial focusing and label-free sorting based on cell size and rigidity, separating CTCs from >95% of white blood cells [4].
- CTC Enumeration & Staining:
- Immunofluorescence staining: Identify CTCs as nucleated (DAPI+), epithelial (pancytokeratin, panCK+), and leukocyte-deficient (CD45-) cells [4].
- Single-Cell RNA Sequencing:
- Pool enriched cells and load onto a single-cell platform (e.g., 10x Genomics Chromium Flex). Generate barcoded, sequence-ready libraries per the manufacturer's protocol [4].
- In parallel, process a matched formalin-fixed, paraffin-embedded (FFPE) tumor tissue scroll for scRNA-seq to enable comparison between CTCs and the primary tumor [4].
- Downstream Analysis: Perform bioinformatic analyses for clustering, differential expression, pathway analysis (e.g., EMT, immunoregulatory pathways), and clonal relationship inference [4].
Extracellular Vesicles (EVs)
Extracellular Vesicles (EVs) are heterogeneous, lipid bilayer-enclosed nanoparticles released by cells, including exosomes and microvesicles. They carry a diverse molecular cargo (proteins, lipids, DNA, various RNA species) reflective of their cell of origin, and play critical roles in cell-cell communication within the tumor microenvironment [5].
Table 3: Biomarker Cargo of Extracellular Vesicles in Cancer
| EV Cargo Type | Example Biomarkers | Potential Clinical Utility |
|---|---|---|
| Proteins | EGFR, PD-L1, Glypican-1 [5] | Diagnosis, therapy monitoring |
| microRNA (miRNA) | miR-21, miR-141 [5] | Early detection, prognosis |
| Long Non-Coding RNA (lncRNA) | MALAT1, HOTAIR [5] | Disease progression, metastasis |
| Circular RNA (circRNA) | cir-ITCH, CDR1as [5] | Biomarker panels |
| DNA | gDNA, mtDNA fragments [5] | Mutational profiling |
Protocol 3: EV Isolation and Cargo Analysis from Plasma
- Sample Preparation: Centrifuge blood plasma (2,000 × g for 20 min) to remove cells and debris. Collect the supernatant for EV isolation.
- EV Isolation: Use one of the following methods, noting that standardization is a key challenge in the field [5]:
- Size-Exclusion Chromatography (SEC): Separates EVs from soluble proteins based on size. Preserves EV integrity and function.
- Ultracentrifugation: The historical gold standard; involves high-speed centrifugation to pellet EVs.
- Precipitation Kits: Polymer-based kits that co-precipitate EVs. Can be user-friendly but may co-precipitate non-EV material.
- Characterization:
- Nanoparticle Tracking Analysis (NTA): Determines EV particle size distribution and concentration.
- Transmission Electron Microscopy (TEM): Visualizes EV morphology.
- Western Blot: Detects presence of EV marker proteins (e.g., CD9, CD63, CD81) and absence of negative markers (e.g., GM130).
- Cargo Profiling:
- RNA Analysis: Extract total RNA from EVs. For miRNA, use specific RT-qPCR panels or small RNA sequencing. For other RNAs, use RNA sequencing.
- Protein Analysis: Perform proteomic analysis via mass spectrometry or multiplex immunoassays (e.g., Luminex) to identify tumor-associated proteins [5].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 4: Key Reagent Solutions for Liquid Biopsy Research
| Reagent/Material | Function | Example Use Case |
|---|---|---|
| Cell-Free DNA BCT Tubes | Stabilizes nucleated cells & cfDNA for extended storage/transport. | Preserves sample integrity in multi-center trials [4]. |
| Silica-Membrane/ Magnetic Bead Kits | Efficient isolation of high-quality cfDNA from plasma. | Input material for all downstream ctDNA assays [1]. |
| Unique Molecular Identifiers (UMIs) | Molecular barcodes for error correction in NGS. | Essential for distinguishing true low-frequency variants [1]. |
| Hybrid-Capture or PCR Panels | Target enrichment for sequencing. | Tumor-informed MRD detection & tumor-agnostic profiling [2] [1]. |
| EpCAM-Coated Magnetic Beads | Immuno-affinity capture of epithelial CTCs. | CTC enumeration using CellSearch system [3]. |
| Microfluidic Labyrinth Chip | Label-free, size-based enrichment of CTCs. | Isolation of viable CTCs for functional studies & scRNA-seq [4]. |
| Fixation/Permeabilization Buffers | Prepare cells for intracellular & nuclear staining. | Enables AR-V7 detection in CTCs from mCRPC [3]. |
| Single-Cell Library Prep Kits | Generate barcoded NGS libraries from single cells. | Molecular profiling of CTCs & matched tumor tissue [4]. |
| Size-Exclusion Chromatography Columns | Isolate intact EVs from biofluids based on size. | Preparation of pure EV samples for downstream cargo analysis [5]. |
| EV Lysis Buffer | Break lipid bilayer to release intravesicular content. | Required for RNA/protein extraction from isolated EVs [5]. |
| Bayothrin | Bayothrin (Transfluthrin) | Bayothrin (Transfluthrin) is a chiral pyrethroid insecticide for research. This product is for Research Use Only (RUO), not for human or veterinary use. |
| Ondansetron-13C,d3 | Ondansetron-13C,d3, MF:C18H19N3O, MW:297.37 g/mol | Chemical Reagent |
Circulating tumor DNA (ctDNA) refers to the fraction of cell-free DNA (cfDNA) in the bloodstream that originates from tumor cells. As a cornerstone of liquid biopsy, ctDNA provides a non-invasive window into tumor biology, enabling researchers and clinicians to assess tumor burden, genetic heterogeneity, and treatment response in real-time. The biological properties of ctDNA—including its origins, shedding mechanisms, and rapid clearance—underpin its value as a dynamic biomarker in cancer research and clinical development. This document details the core biology of ctDNA and provides standardized protocols for its study, supporting its integration into robust liquid biopsy workflows for cancer monitoring.
Biological Foundations of ctDNA
Cellular Origins and Mechanisms of Release
ctDNA is released into the bloodstream primarily through apoptosis and necrosis of tumor cells [1] [6]. During apoptosis, controlled enzymatic cleavage results in short DNA fragments, while necrosis releases longer, more variable fragments due to uncontrolled cell death.
- Primary Source: The majority of ctDNA originates from tumor cells undergoing programmed cell death (apoptosis) [1] [6].
- Fragment Characteristics: ctDNA fragments typically exhibit a characteristic size profile of 90–150 base pairs, which is often shorter than the cfDNA derived from healthy cell apoptosis [2] [6]. This size difference can be leveraged in laboratory assays to enrich for tumor-derived material.
- Influencing Factors: The amount of ctDNA shed is influenced by tumor vascularity and biological aggressiveness. Tumors with greater vascular invasion tend to release more ctDNA [6]. The concentration of ctDNA in plasma generally correlates with tumor burden, though this relationship can vary significantly across cancer types and individual patients [1].
Half-Life and Clearance Dynamics
A critical property of ctDNA is its remarkably short half-life in circulation, which enables near real-time monitoring of tumor dynamics.
- Clearance Rate: The half-life of ctDNA is estimated to be between 16 minutes and 2.5 hours [1] [7]. This rapid clearance allows researchers to observe molecular changes in the tumor landscape within hours of an intervention.
- Clearance Mechanisms: ctDNA is rapidly eliminated from the bloodstream by liver macrophages (Kupffer cells) and through degradation by circulating nucleases [6].
Table 1: Key Biological Characteristics of ctDNA
| Biological Feature | Description | Research Implication |
|---|---|---|
| Primary Origin | Apoptosis and necrosis of tumor cells [1] [6] | Reflects tumor cell turnover rate |
| Typical Fragment Size | 90–150 base pairs [2] [6] | Enables size-selection enrichment strategies |
| Half-Life in Circulation | 16 minutes to 2.5 hours [1] [7] | Allows for real-time disease monitoring |
| Clearance Mechanism | Phagocytosis by liver macrophages and degradation by circulating nucleases [6] | Physiological processes that limit detection windows |
Figure 1: ctDNA Lifecycle. This diagram illustrates the release of ctDNA into the bloodstream primarily through apoptosis and necrosis of tumor cells, followed by its rapid clearance via hepatic macrophages and circulating nucleases.
Quantitative Assessment of ctDNA in Research
Prognostic Value Across the Treatment Continuum
The presence and concentration of ctDNA at key clinical timepoints provide powerful prognostic information across multiple cancer types.
Table 2: Prognostic Value of ctDNA at Different Timepoints (Meta-Analysis Data from Esophageal Cancer) [8]
| Timepoint of ctDNA Detection | Hazard Ratio (HR) for Progression-Free Survival | Hazard Ratio (HR) for Overall Survival |
|---|---|---|
| Baseline (After diagnosis, before treatment) | HR = 1.64 (95% CI: 1.30–2.07) | HR = 2.02 (95% CI: 1.36–2.99) |
| Post-Neoadjuvant Therapy (After therapy, before surgery) | HR = 3.97 (95% CI: 2.68–5.88) | HR = 3.41 (95% CI: 2.08–5.59) |
| During Follow-up (Monitoring phase) | HR = 5.42 (95% CI: 3.97–7.38) | HR = 4.93 (95% CI: 3.31–7.34) |
Key findings from this meta-analysis indicate that a positive ctDNA test is associated with a poorer prognosis throughout the patient's treatment journey, and the prognostic value of ctDNA status increases over time, from baseline through follow-up [8]. Furthermore, ctDNA-based detection can predict clinical recurrence an average of 4.53 months earlier (range: 0.98–11.6 months) than conventional radiological imaging [8].
Experimental Protocols for ctDNA Analysis
Pre-Analytical Phase: Blood Collection and Plasma Processing
The pre-analytical phase is critical for preserving ctDNA integrity, as improper handling can lead to contamination from genomic DNA released by lysed blood cells.
Protocol: Blood Collection and Plasma Separation
- Blood Draw: Collect venous blood using cfDNA BCT blood collection tubes (e.g., from Streck, Qiagen, or Roche), which contain preservatives to stabilize nucleated blood cells and prevent lysis. This allows for sample storage and transport at room temperature for up to 7 days [6]. If using conventional EDTA tubes, process the blood within 2–6 hours of collection [6].
- Plasma Separation: Perform a two-step centrifugation protocol.
- First Centrifugation: Centrifuge the blood tube at 800–1,600 × g for 10 minutes at 4°C to separate plasma from blood cells.
- Plasma Transfer: Carefully transfer the supernatant (plasma) to a new tube, avoiding the buffy coat and cell pellet.
- Second Centrifugation: Centrifuge the plasma again at 16,000 × g for 10 minutes at 4°C to remove any remaining cellular debris.
- Storage: Aliquot the purified plasma and store at -80°C until cfDNA extraction is performed.
Analytical Phase: ctDNA Detection and Quantification
Protocol: Tumor-Informed ctDNA Detection using NGS
This protocol uses next-generation sequencing (NGS) for highly sensitive detection of ctDNA, informed by prior sequencing of tumor tissue.
- DNA Extraction: Extract total cfDNA from plasma using commercial kits (e.g., QIAamp Circulating Nucleic Acid Kit). Quantify the yield using fluorometry.
- Tumor Tissue Sequencing: For the tumor-informed approach, first sequence the patient's tumor tissue (e.g., from a biopsy or resection) using Whole Exome Sequencing (WES) to identify patient-specific somatic mutations (SNVs, indels) [9].
- Custom Panel Design: Design a custom NGS panel targeting ~150 personalized somatic variants identified from the tumor tissue [9].
- Library Preparation & Sequencing: Prepare sequencing libraries from the plasma cfDNA using the custom panel. Incorporate Unique Molecular Identifiers (UMIs) to tag individual DNA molecules before amplification, enabling bioinformatic correction of PCR and sequencing errors [1].
- Bioinformatic Analysis: Process the sequencing data through a pipeline that includes:
- UMI Consensus Calling: Group reads originating from the same original DNA molecule to create a consensus sequence and eliminate random errors.
- Variant Calling: Identify somatic variants present in the plasma cfDNA.
- Variant Allele Frequency (VAF) Calculation: Determine the fraction of DNA molecules in the plasma that carry the tumor-specific mutation.
- Result Interpretation: A positive ctDNA signal is defined by the detection of tumor-specific mutations above the assay's limit of detection (LOD), which for advanced assays can be as low as 0.002% VAF [9].
Figure 2: Tumor-Informed ctDNA Analysis Workflow. The process begins with blood draw and plasma processing, followed by cfDNA extraction. A custom sequencing panel is designed based on mutations found in tumor tissue WES. Plasma cfDNA is then sequenced using this panel, and data is analyzed with UMI-error correction.
The Scientist's Toolkit: Essential Research Reagents and Platforms
Table 3: Key Research Reagent Solutions for ctDNA Analysis
| Reagent / Platform | Primary Function | Key Feature in Research |
|---|---|---|
| cfDNA BCT Tubes (Streck, Qiagen, Roche) [6] | Stabilizes blood cells during storage/transport | Prevents genomic DNA contamination; enables multi-day shipping |
| Unique Molecular Identifiers (UMIs) [1] | Molecular barcoding of DNA fragments | Enables error correction and accurate quantification of low-frequency variants |
| Custom Target Enrichment Panels (e.g., VIGIL) [9] | NGS panel for targeted sequencing | Allows highly sensitive (LOD ~0.002%) tumor-informed ctDNA tracking |
| Methylation-Specific Assays (e.g., Northstar Response) [10] | Quantifies ctDNA via cancer-associated methylation | Tumor-naive approach; tracks abundant epigenetic changes |
| Digital PCR (dPCR/ddPCR) [1] [7] | Absolute quantification of known mutations | High sensitivity for tracking specific mutations without NGS |
| Imiquimod-d9 | Imiquimod-d9, MF:C14H16N4, MW:249.36 g/mol | Chemical Reagent |
| Abemaciclib metabolite M18 hydrochloride | Abemaciclib metabolite M18 hydrochloride, MF:C25H29ClF2N8O, MW:531.0 g/mol | Chemical Reagent |
The fundamental biology of ctDNA—its origin from apoptotic tumor cells, its characteristic fragment size, and its rapid clearance from the bloodstream—makes it an exceptionally powerful tool for cancer monitoring in research and drug development. The protocols and tools detailed in this document provide a framework for implementing robust ctDNA analysis, paving the way for advancements in minimal residual disease detection, therapy response monitoring, and the development of novel cancer therapeutics.
Circulating tumor cells (CTCs) are cancer cells that detach from primary or metastatic tumors and enter the bloodstream, serving as crucial mediators of cancer metastasis. As a key component of liquid biopsy, CTC analysis provides a minimally invasive approach for cancer diagnosis, prognosis assessment, treatment monitoring, and understanding metastasis mechanisms. This application note examines the technical challenges in CTC isolation and detection, explores established and emerging methodologies, and discusses the clinical validation and implementation of CTC enumeration across various cancer types. We present standardized protocols for CTC analysis using both commercial and research platforms, along with practical considerations for integrating CTC assessment into cancer management workflows.
Circulating tumor cells (CTCs) are cancer cells that shed from primary and metastatic tumor sites into the bloodstream or lymphatic system [11] [12]. First identified by Ashworth in 1869, CTCs have emerged as a critical biomarker in oncology, providing insights into tumor biology and metastasis [11] [13]. As seeds of metastasis, CTCs undergo a complex cascade involving dissemination, survival in circulation, extravasation, and colonization of distant organs [13]. The detection and analysis of CTCs offer a real-time, minimally invasive "liquid biopsy" that can complement or potentially replace traditional tissue biopsies in certain clinical scenarios [11] [14].
CTCs exhibit remarkable heterogeneity, with subpopulations undergoing epithelial-to-mesenchymal transition (EMT), a process that enhances cell motility and invasiveness [11] [13]. During EMT, CTCs often downregulate epithelial markers such as EpCAM and upregulate mesenchymal markers like vimentin and twist, complicating their detection [13] [14]. Additionally, CTCs can display stem-like properties, interact with various blood components (platelets, neutrophils), and form clusters, all of which contribute to their metastatic potential and survival in the harsh circulatory environment [11] [12].
The clinical significance of CTCs has been demonstrated across multiple cancer types. In metastatic breast, prostate, and colorectal cancers, CTC enumeration has prognostic value and can inform treatment decisions [3]. Regulatory approvals, including the FDA clearance of the CellSearch system for metastatic breast, prostate, and colorectal cancers, have established CTCs as clinically relevant biomarkers [3]. Recent expert consensus confirms the clinical utility of CTCs for prognosis and treatment monitoring in metastatic breast and prostate cancers, including AR-V7 testing in metastatic castration-resistant prostate cancer for therapy selection [3].
Challenges in CTC Isolation and Detection
The isolation and detection of CTCs present significant technical challenges primarily due to their extreme rarity and heterogeneity within blood samples.
Table 1: Key Challenges in CTC Detection and Potential Solutions
| Challenge | Explanation | Potential Solutions | References |
|---|---|---|---|
| Rarity | As few as 1 CTC per billion blood cells; fewer than 1 CTC per 10 mL in early-stage cancer | Efficient enrichment methods; depletion of unwanted cells; processing large blood volumes | [11] [12] |
| Heterogeneity | Phenotypic and genotypic variations; marker expression differences (e.g., EpCAM downregulation during EMT) | Multiple biomarkers; combined size- and label-based approaches; label-free detection | [11] [13] |
| Complex Blood Environment | Non-specific binding from blood cells and plasma proteins creates high background noise | Antifouling surfaces; improved surface-cell interaction; dual-selective ligands | [11] |
| Low Viability of Captured CTCs | Shear stress during isolation causes apoptosis/necrosis; delays in processing affect downstream analysis | Gentle capture via microfluidics; low-shear designs; integrated culture systems | [11] |
| Clinical Implementation | Rapid processing needed; short storage times; logistical hurdles; high device costs | Preservative tubes; automated capture and analysis; standardized protocols | [11] [3] |
Biological and Technical Complexities
The rarity of CTCs represents a fundamental detection challenge, with concentrations as low as 1-10 CTCs per milliliter of whole blood amid approximately 10 million leukocytes and 5 billion erythrocytes [11]. This scarcity necessitates highly sensitive detection methods and efficient enrichment strategies. Furthermore, CTCs display considerable heterogeneity in size, shape, surface marker expression, and mechanical properties, complicating their isolation using standardized parameters [11] [12].
The epithelial-mesenchymal transition (EMT) process particularly impacts detection efficiency. As CTCs undergo EMT, they downregulate epithelial markers such as EpCAM, which forms the basis of many capture technologies [13]. This biological plasticity can lead to false negatives when relying solely on epithelial markers for detection. Physical properties of CTCs, including larger size (typically 12-25 μm compared to 8-12 μm for leukocytes) and increased nuclear-to-cytoplasmic ratio, provide alternative separation parameters but also show considerable variability across cancer types and individuals [11] [12].
The blood microenvironment further complicates CTC detection through non-specific binding of blood cells and plasma proteins, creating background noise that obscures signal detection. Additionally, maintaining CTC viability post-capture remains challenging but crucial for downstream functional analyses and culture [11].
Established CTC Enumeration Methods and Clinical Validation
CTC enumeration has achieved the highest level of clinical validation in metastatic cancers, with technologies now incorporated into clinical practice for specific indications.
Table 2: Clinical Applications of CTC Enumeration Across Cancer Types
| Cancer Type | Clinical Utility | Evidence Level | Regulatory Status | References |
|---|---|---|---|---|
| Metastatic Breast Cancer | Prognosis, treatment monitoring | High | FDA-cleared (CellSearch) | [3] |
| Metastatic Prostate Cancer | Prognosis, treatment monitoring; AR-V7 for therapy selection | High | FDA-cleared (CellSearch) | [3] |
| Metastatic Colorectal Cancer | Prognosis | High | FDA-cleared (CellSearch) | [3] [15] |
| Early-Stage Breast Cancer | Minimal residual disease detection (promising) | Moderate | Investigational | [3] [16] |
| Other Solid Tumors | Prognosis, treatment monitoring | Variable | Investigational | [3] |
Clinically Validated Platforms and Technologies
The CellSearch system represents the first and only FDA-cleared technology for clinical CTC enumeration in metastatic breast, prostate, and colorectal cancers [3] [17]. This system uses immunomagnetic enrichment with anti-EpCAM antibodies followed by fluorescent staining for cytokeratins (CK 8, 18, 19), CD45 (leukocyte marker), and DAPI (nuclear stain) to identify and enumerate CTCs [17]. Clinical studies have consistently demonstrated that elevated CTC counts (≥5 CTCs/7.5 mL blood in breast cancer) correlate with poorer progression-free and overall survival [3].
Beyond enumeration, molecular characterization of CTCs provides additional clinical value. In metastatic castration-resistant prostate cancer, detection of the AR-V7 splice variant in CTCs predicts resistance to androgen receptor signaling inhibitors and helps guide therapy selection toward taxane-based chemotherapy [3]. Similarly, HER2 status assessment in CTCs from breast cancer patients can inform targeted therapy decisions, particularly when tissue biopsy is unavailable or discordant [3].
Recent international expert consensus confirms that CTCs have established clinical utility in metastatic breast and prostate cancers, while their application in other settings remains investigational [3]. The consensus emphasizes the need to shift from simple enumeration to phenotypic and molecular characterization while integrating CTC analysis with other liquid biopsy components like cell-free DNA for comprehensive cancer monitoring [3].
Experimental Protocols
This section provides detailed methodologies for CTC isolation, enumeration, and characterization using established platforms and emerging technologies.
Protocol: CTC Enumeration Using CellSearch System
Principle
The CellSearch system isolates and enumerates CTCs from whole blood through immunomagnetic enrichment targeting epithelial cell adhesion molecule (EpCAM), followed by immunofluorescent staining to identify nucleated cells expressing epithelial markers but lacking leukocyte markers [17].
Materials and Reagents
- CellSearch Circulating Tumor Cell Kit (includes staining reagents, capture beads, and sample tubes)
- CellSave Preservative Tubes (10 mL)
- CellSearch AutoPrep System
- CellSearch Analyzer II
- Phosphate-buffered saline (PBS)
- Isopropanol (70%)
- Deionized water
Procedure
Blood Collection and Storage: Collect 10 mL peripheral blood into CellSave tubes. Invert tubes gently 8-10 times to mix preservative. Store at room temperature and process within 96 hours of collection.
Sample Preparation: Place sample tube into CellSearch AutoPrep system along with necessary reagents. The system automatically performs:
- Plasma separation by centrifugation
- Immunomagnetic enrichment with ferrofluid nanoparticles conjugated with anti-EpCAM antibodies
- Transfer of labeled cells to a cartridge chamber
- Permeabilization and staining with fluorescent antibodies:
- Phycoerythrin-conjugated anti-cytokeratin (CK 8,18,19) antibodies
- Allophycocyan-conjugated anti-CD45 antibody
- 4',6-diamidino-2-phenylindole (DAPI) nuclear stain
Image Acquisition and Analysis: The prepared cartridge is transferred to the CellSearch Analyzer, which:
- Scans the entire cartridge surface using a semi-automated fluorescence microscope
- Captures images in four fluorescence channels
- Identifies potential CTCs based on predefined criteria:
- DAPI positive (nucleated)
- Cytokeratin positive (epithelial origin)
- CD45 negative (non-hematopoietic)
- Morphologically consistent with tumor cells
Interpretation and Enumeration: A trained technician reviews the system-identified events to confirm CTC classification. CTCs are defined as nucleated cells expressing cytokeratin but lacking CD45. Results are reported as number of CTCs per 7.5 mL of blood.
Quality Control
- Process control samples with each batch to ensure proper system performance
- Monitor antibody fluorescence intensity and magnetic capture efficiency
- Maintain standardized imaging and analysis parameters
Protocol: Microfluidic CTC Capture Using Chip-Based Platforms
Principle
Microfluidic CTC isolation utilizes precisely engineered chips containing microstructures or patterns that enhance interactions between CTCs and capture surfaces, either through affinity-based methods (antibody-functionalized surfaces) or size-based separation [11].
Materials and Reagents
- Microfluidic chip (e.g., HB-Chip, graphene oxide chip, or similar)
- Syringe pump with precise flow control
- Antibodies for surface functionalization (e.g., anti-EpCAM, anti-HER2)
- Phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA)
- Fixation solution (4% paraformaldehyde)
- Permeabilization buffer (0.1% Triton X-100 in PBS)
- Fluorescently labeled antibodies for characterization
- Vacuum-driven tubing and connectors
Procedure
Chip Preparation and Functionalization:
- Clean chip surface with 70% ethanol followed by PBS
- Incubate with selected capture antibodies (10-50 μg/mL in PBS) for 2 hours at room temperature or overnight at 4°C
- Block non-specific binding sites with 1% BSA in PBS for 1 hour
- Rinse with PBS to remove unbound antibodies
Blood Sample Preparation:
- Collect peripheral blood in anticoagulant tubes (EDTA or citrate)
- Dilute blood 1:1 with PBS containing 1% BSA
- Filter through 40 μm cell strainer to remove large aggregates
Microfluidic Processing:
- Load prepared chip onto microscope stage if real-time monitoring is desired
- Connect chip to syringe pump using appropriate tubing
- Prime chip with PBS at low flow rate (0.5 mL/hour)
- Introduce diluted blood sample at optimized flow rate (1-2 mL/hour)
- Wash with 10-15 mL PBS at 1 mL/hour to remove unbound cells
On-Chip Staining and Analysis:
- Fix cells with 4% paraformaldehyde for 15 minutes
- Permeabilize with 0.1% Triton X-100 for 10 minutes if intracellular staining required
- Stain with fluorescent antibodies for identification (e.g., anti-cytokeratin, anti-CD45)
- Counterstain with DAPI for nucleus visualization
- Image directly on chip using fluorescence microscopy
Cell Recovery (if needed for downstream analysis):
- For live cell recovery: introduce enzyme solution (e.g., trypsin) to release captured cells
- Collect effluent in small volumes and centrifuge to concentrate cells
- Plate for culture or process for molecular analysis
Quality Control
- Validate chip efficiency using spiked tumor cell lines before patient samples
- Monitor flow rates consistently to ensure reproducible performance
- Include negative controls (healthy donor blood) to assess non-specific binding
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents for CTC Isolation and Analysis
| Reagent/Material | Function | Application Examples | References |
|---|---|---|---|
| Anti-EpCAM Antibodies | Immunoaffinity capture of epithelial CTCs | CellSearch system; magnetic bead-based isolation; microfluidic chip coating | [11] [17] |
| Anti-CD45 Antibodies | Negative selection; leukocyte depletion | Negative enrichment strategies; identification of hematopoietic contamination | [17] |
| Cytokeratin Antibodies (CK 8,18,19) | CTC identification via intermediate filament proteins | Immunofluorescence staining for CTC confirmation | [17] |
| DAPI (4',6-diamidino-2-phenylindole) | Nuclear staining for cell identification | Distinguishing nucleated cells from debris; viability assessment | [17] |
| CellSave Preservative Tubes | Blood collection and stabilization | Maintains CTC integrity for up to 96 hours post-collection | [15] |
| Immunomagnetic Beads | Magnetic separation of labeled cells | Positive (EpCAM+) or negative (CD45+) enrichment of CTC populations | [17] |
| Microfluidic Chips | Miniaturized platforms for CTC capture | HB-Chip; graphene oxide chips; size-based isolation devices | [11] |
| Membrane Filters (Polycarbonate) | Size-based separation of CTCs | Isolation by Size of Epithelial Tumor cells (ISET) | [14] |
| (Rac)-OSMI-1 | (Rac)-OSMI-1, MF:C28H25N3O6S2, MW:563.6 g/mol | Chemical Reagent | Bench Chemicals |
| Teicoplanin A2-5 | Teicoplanin A2-5, MF:C89H99Cl2N9O33, MW:1893.7 g/mol | Chemical Reagent | Bench Chemicals |
CTC Metastasis Pathway and Detection Workflow
The following diagrams illustrate key biological and technical aspects of CTC analysis.
CTC Metastasis Cascade
CTC Detection Workflow
CTC analysis represents a transformative approach in cancer management, bridging basic research and clinical application. While significant progress has been made in standardization and validation of CTC enumeration for prognostic applications in metastatic cancers, ongoing research focuses on expanding clinical utility through molecular characterization, integration with other liquid biopsy components, and technological innovations enhancing detection sensitivity. The protocols and methodologies outlined in this application note provide a foundation for implementing CTC analysis in cancer research and clinical practice, with the potential to significantly impact personalized cancer care through non-invasive monitoring of disease progression and treatment response.
Liquid biopsy represents a transformative, minimally invasive approach in oncology, enabling real-time detection and monitoring of cancer through the analysis of circulating tumor DNA (ctDNA) and other blood-borne biomarkers [18]. Among the most promising analytical targets are epigenetic markers, particularly DNA methylation and non-coding RNAs (ncRNAs), which provide critical insights into tumor heterogeneity, evolution, and therapeutic response [18] [19]. These epigenetic alterations often emerge early in tumorigenesis and remain stable throughout disease progression, making them ideal biomarkers for early cancer detection, prognosis assessment, and monitoring minimal residual disease (MRD) [20] [19].
The integration of epigenetic markers with advanced detection technologies and artificial intelligence is paving the way for individualized, dynamically guided oncology care, moving beyond the capabilities of traditional tissue biopsies [18] [21]. This document provides detailed application notes and experimental protocols for investigating these emerging epigenetic markers within the context of a comprehensive liquid biopsy workflow for cancer monitoring research.
Emerging Epigenetic Marker Profiles
DNA Methylation Biomarkers
DNA methylation involves the covalent addition of a methyl group to the 5-position of cytosine within CpG dinucleotides, predominantly in CpG islands located in gene promoter regions [18] [20]. In cancer, aberrant methylation patterns include hypermethylation of tumor suppressor genes and global hypomethylation that can lead to genomic instability [18] [20]. The stability of DNA methylation patterns in circulating cell-free DNA (cfDNA) and their early emergence in carcinogenesis make them particularly valuable for clinical applications [20] [19].
Table 1: DNA Methylation Biomarkers in Cancer Detection and Monitoring
| Cancer Type | Methylation Biomarkers | Sample Type | Clinical Application | Performance Characteristics | References |
|---|---|---|---|---|---|
| Lung Cancer | SHOX2, RASSF1A, PTGER4 | Plasma, Bronchoalveolar Lavage Fluid | Early detection, diagnosis | Detection of early-stage disease | [22] |
| Colorectal Cancer | SDC2, SEPT9, SFRP2 | Plasma, Stool | Early screening, monitoring | Sensitivity: 86.4%, Specificity: 90.7% | [22] |
| Breast Cancer | TRDJ3, PLXNA4, KLRD1, KLRK1 | PBMCs, Plasma | Early detection | Sensitivity: 93.2%, Specificity: 90.4% | [22] |
| Hepatocellular Carcinoma | SEPT9, BMPR1A, PLAC8 | Plasma | Detection and monitoring | Correlates with tumor burden | [22] |
| Pan-Cancer | Multi-gene methylation panels | Plasma | Multi-cancer early detection | High specificity (98.5%), variable sensitivity | [16] [23] |
Non-Coding RNA Biomarkers
Non-coding RNAs represent a diverse class of regulatory RNAs that include microRNAs (miRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs), and other subtypes [24] [25]. These molecules are remarkably stable in biofluids due to protection within extracellular vesicles, lipoprotein complexes, or protein complexes, making them excellent biomarker candidates [24] [25]. They play crucial roles in cancer progression through regulation of gene expression at transcriptional and post-transcriptional levels.
Table 2: Non-Coding RNA Biomarkers in Liquid Biopsy
| RNA Category | Representative Biomarkers | Cancer Type | Biological Function | Clinical Utility | References |
|---|---|---|---|---|---|
| miRNA | miR-21, miR-34a, miR-205-5p | Multiple cancers | Oncogenic, tumor suppressive | Treatment response monitoring, prognosis | [18] [25] |
| lncRNA | HOTAIR, MALAT1, NEAT1 | Multiple cancers | Chromatin remodeling, metastasis | Disease progression, metastasis detection | [18] [25] |
| circRNA | Various tissue-specific | Multiple cancers | miRNA sponges, regulatory functions | Early detection, monitoring | [24] [25] |
| OncRNA | T3p, lung cancer-emergent oncRNAs | Non-small Cell Lung Cancer | Cancer-emergent small RNAs | Early detection (94% sensitivity) | [21] |
| piRNA | Various | Germ cell tumors, somatic cancers | Transposon silencing, gene regulation | Emerging diagnostic potential | [24] |
Experimental Protocols
DNA Methylation Analysis Workflow
Sample Collection and Processing
Protocol: Blood Collection and Plasma Separation for Methylation Analysis
- Collect peripheral blood (10-20 mL) in EDTA or Streck Cell-Free DNA BCT tubes to prevent nucleases degradation [20] [26].
- Process within 2-6 hours of collection: centrifuge at 800-1600 × g for 10 minutes at 4°C to separate plasma from peripheral blood mononuclear cells (PBMCs) [20].
- Transfer supernatant to fresh tubes and perform a second centrifugation at 16,000 × g for 10 minutes to remove residual cells [20] [26].
- Store plasma at -80°C until cfDNA extraction. Avoid freeze-thaw cycles.
cfDNA Extraction and Bisulfite Conversion
Protocol: cfDNA Isolation and Bisulfite Treatment
- Extract cfDNA from 1-5 mL plasma using commercial kits (QIAamp Circulating Nucleic Acid Kit, MagMAX Cell-Free DNA Isolation Kit) with carrier RNA to improve recovery [22] [26].
- Quantify cfDNA using fluorometric methods (Qubit dsDNA HS Assay); expected yield: 5-50 ng from 1 mL plasma [26].
- Convert 10-50 ng cfDNA using bisulfite treatment kits (Zymo Research EZ DNA Methylation, Qiagen EpiTect Fast DNA Bisulfite) following manufacturer protocols [22] [26].
- Critical Step: Optimize conversion conditions to minimize DNA fragmentation while ensuring complete conversion (>99% efficiency) [22].
Methylation Detection and Analysis
Protocol: Targeted Methylation Sequencing
- For genome-wide analysis: Use Whole-Genome Bisulfite Sequencing (WGBS) or Reduced Representation Bisulfite Sequencing (RRBS) for discovery phases [20] [22].
- For clinical validation: Employ targeted approaches like bisulfite sequencing with custom panels or methylation-specific PCR [20] [19].
- Library preparation: Use kits compatible with bisulfite-converted DNA (Accel-NGS Methyl-Seq DNA Library Kit) with unique molecular identifiers (UMIs) to reduce PCR duplicates [22] [26].
- Sequencing: Minimum 50,000x coverage for targeted panels; appropriate spike-in controls (EpiCypher SNAP controls) for quality monitoring [26].
- Bioinformatics: Align to bisulfite-converted reference genome; use specialized tools (Bismark, MethylKit) for methylation calling; account for cfDNA fragmentation patterns [20] [22].
Non-Coding RNA Analysis Workflow
Sample Collection and RNA Stabilization
Protocol: Blood Collection for ncRNA Analysis
- Collect blood in PAXgene Blood RNA tubes or EDTA tubes with RNA stabilization additives [24] [25].
- Process within 2 hours for EDTA tubes; PAXgene tubes can be stored for up to 5 days at room temperature.
- For plasma separation: Follow similar protocol as for DNA methylation analysis with addition of RNase inhibitors to collection tubes [24] [21].
- For serum samples: Allow blood to clot for 30 minutes at room temperature before centrifugation [25].
RNA Extraction from Biofluids
Protocol: Cell-free RNA Extraction
- Extract total cell-free RNA from 0.5-4 mL plasma/serum using commercial kits (miRNeasy Serum/Plasma Kit, exoRNeasy Maxi Kit for exosomal RNA) [24] [21].
- Critical Consideration: Include spike-in synthetic RNAs (e.g., cel-miR-39) for normalization and quality control [24] [26].
- Elute in 14-30 µL nuclease-free water; store at -80°C.
- Quantify using capillary electrophoresis (Agilent Bioanalyzer Small RNA Kit) to assess RNA integrity; expected profile shows predominant small RNA fragments (<200 nt) [21].
Library Preparation and Sequencing
Protocol: Small RNA Sequencing
- Use 1-10 ng total RNA for library preparation with specialized small RNA kits (NEBNext Small RNA Library Prep Set) [24] [21].
- Include unique molecular identifiers (UMIs) to account for PCR amplification bias and enable accurate quantification [21] [26].
- Size selection (140-160 bp for miRNAs, variable for other ncRNAs) using gel electrophoresis or magnetic beads [24].
- Sequence on appropriate platforms (Illumina NextSeq, NovaSeq) with minimum 5-10 million reads per sample for adequate coverage [21].
Bioinformatics Analysis
Protocol: ncRNA Data Analysis
- Quality control: FastQC, MultiQC with adapter trimming [21].
- Alignment: Map to reference genome (GRCh38) with specialized aligners (STAR, Bowtie) accounting for small RNA species [21].
- Quantification: Count reads for known ncRNAs using miRBase, NONCODE, circBase databases [24] [21].
- Novel ncRNA discovery: Use specialized tools (CPC2, FEELnc) for identifying unannotated transcripts [21].
- Differential expression: DESeq2, edgeR with appropriate multiple testing correction [21] [25].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Essential Research Reagents for Epigenetic Liquid Biopsy Analysis
| Reagent Category | Specific Products | Manufacturer Examples | Application Notes |
|---|---|---|---|
| Blood Collection Tubes | Cell-Free DNA BCT tubes, PAXgene Blood RNA tubes | Streck, Qiagen, BD | Preserve nucleic acid integrity during storage and transport |
| Nucleic Acid Extraction Kits | QIAamp Circulating Nucleic Acid Kit, miRNeasy Serum/Plasma Kit | Qiagen, Norgen Biotek | Optimized for low-abundance cfDNA/cfRNA from biofluids |
| Bisulfite Conversion Kits | EZ DNA Methylation Kit, EpiTect Fast DNA Bisulfite Kit | Zymo Research, Qiagen | Critical for DNA methylation analysis; conversion efficiency >99% |
| Library Preparation Kits | Accel-NGS Methyl-Seq, NEBNext Small RNA Library Prep | Swift Biosciences, NEB | Designed for bisulfite-converted DNA or small RNA inputs |
| Spike-in Controls | SNAP Spike-in Controls, synthetic RNA spike-ins | EpiCypher, Lexogen | Quality control and normalization standards |
| Target Enrichment Panels | Custom methylation panels, ncRNA capture panels | Agilent, IDT, Twist Bioscience | Focus sequencing power on clinically relevant targets |
| Quality Control Kits | Bioanalyzer RNA/DNA kits, Qubit assays | Agilent, Thermo Fisher | Essential for input quantification and quality assessment |
| Rock2-IN-2 | Rock2-IN-2|Selective ROCK2 Inhibitor | Bench Chemicals | |
| Usp7-IN-3 | Usp7-IN-3, MF:C29H31F3N6O3, MW:568.6 g/mol | Chemical Reagent | Bench Chemicals |
Advanced Applications and Integrated Analysis
Multi-Omics Integration in Liquid Biopsy
The combination of DNA methylation and ncRNA biomarkers provides complementary information that enhances diagnostic sensitivity and specificity [18] [26]. DNA methylation offers information about the tissue of origin and tumor suppressor silencing, while ncRNAs reflect real-time regulatory activity and cellular communication [18] [24].
Protocol: Integrated Multi-Omic Analysis
- Process parallel aliquots of the same blood sample for DNA methylation and ncRNA analysis [26].
- Utilize multi-task generative AI models (e.g., Orion) to integrate methylation and ncRNA data while accounting for technical confounders [21].
- Implement cross-validation strategies to assess model performance and generalizability to independent datasets [21].
Clinical Validation and Implementation
For successful translation of epigenetic biomarkers into clinical practice, rigorous validation is essential [20] [23].
Protocol: Analytical Validation
- Determine limit of detection (LOD) using dilution series of methylated DNA or synthetic ncRNA spikes in normal plasma [20] [26].
- Assess precision through repeatability (within-run) and reproducibility (between-run, between-operator, between-laboratory) experiments [20].
- Establish reference ranges using appropriate control populations matched for age, sex, and comorbidities [20].
- For in vitro diagnostic applications, follow FDA, CE-IVD, or other relevant regulatory guidelines [23].
Epigenetic markers in cfDNA, particularly DNA methylation and non-coding RNAs, represent powerful tools for cancer detection and monitoring through liquid biopsy. The protocols outlined herein provide a framework for implementing these analyses in research settings, with potential for translation into clinical practice. As detection technologies continue to advance and computational methods become more sophisticated, these epigenetic biomarkers are poised to play an increasingly important role in personalized oncology, enabling earlier detection, better monitoring, and more tailored therapeutic interventions.
Comparative Analysis of Biomarker Properties and Clinical Applications
Biomarkers are defined as measurable characteristics that serve as indicators of normal biological processes, pathogenic processes, or responses to an exposure or intervention [27]. According to the joint FDA-NIH Biomarkers, EndpointS, and other Tools (BEST) resource, biomarkers are categorized into seven primary classes based on their clinical application: diagnostic, monitoring, pharmacodynamic/response, predictive, prognostic, safety, and susceptibility/risk biomarkers [27] [28]. This classification system provides a critical framework for understanding how different biomarkers contribute to disease management and therapeutic development.
The discovery and validation of biomarkers, particularly from high-dimensional genomic data, typically employs feature selection techniques to identify the most discriminating features for a given classification task [29]. Effective biomarkers share essential characteristics including high sensitivity and specificity, reproducibility across different laboratories and over time, ease of measurement, affordability, consistency across diverse populations, correlation with disease severity, and the ability to provide adequate lead time for early intervention [30]. Furthermore, reliable biomarkers should demonstrate a dynamic response to treatment and possess a clear mechanistic link to the disease process [30].
Table 1: BEST Resource Biomarker Classification Framework
| Biomarker Category | Primary Function | Clinical Context |
|---|---|---|
| Diagnostic | Detect or confirm presence of a disease or condition [27] | Identify individuals with a disease subtype; redefine disease classification [27] |
| Monitoring | Assess status of disease or medical condition serially [27] | Track chronic diseases (e.g., HbA1c in diabetes); measure exposure to medical products [27] |
| Pharmacodynamic/Response | Indicate biological response to therapeutic intervention [28] | Assess pharmacological effects; guide dose selection in early clinical development [28] |
| Predictive | Identify likelihood of response to specific treatment [27] | Select patients for targeted therapies; avoid ineffective treatments and associated toxicity [27] |
| Prognostic | Identify likelihood of clinical event or disease progression [28] | Inform long-term disease management strategies; assess natural history of disease [28] |
| Safety | Indicate likelihood of adverse event or toxicity [27] [28] | Monitor drug safety; detect organ damage or physiological stress [27] |
| Susceptibility/Risk | Identify potential for developing disease or condition [27] | Stratify populations for screening; implement preventive measures for high-risk individuals [27] |
Biomarker Applications in Liquid Biopsy for Cancer Monitoring
Liquid biopsy represents a transformative approach in oncology that analyzes tumor-derived components from bodily fluids, primarily blood, offering a minimally invasive alternative to traditional tissue biopsies [31] [32]. This technology enables serial sampling to monitor tumor evolution, therapeutic response, and emerging resistance mechanisms over time [31]. The continuous perfusion of blood through tumors allows for the collection of various cancer components, including circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), tumor-derived extracellular vesicles (EVs), tumor-educated platelets (TEPs), and circulating cell-free RNA (cfRNA) [31]. These analytes provide complementary information about tumor heterogeneity, mutation profiles, and clonal evolution that is crucial for personalized cancer management.
Liquid biopsy offers significant advantages over tissue biopsy, including minimal invasiveness, rapid turnaround time, accessibility for serial sampling, and the ability to capture tumor heterogeneity more comprehensively [31] [32]. The clinical applications of liquid biopsy in cancer management encompass early detection and screening, prognosis assessment, monitoring treatment response, identifying minimal residual disease (MRD), detecting early relapse, and guiding treatment decisions by identifying actionable mutations [31]. Current research efforts focus on optimizing pre-analytical and analytical processes to enhance the sensitivity and specificity of liquid biopsy platforms, with numerous clinical trials underway to validate its utility in various cancer types [31].
Table 2: Analytical Components in Liquid Biopsy and Their Clinical Applications
| Liquid Biopsy Component | Description | Primary Cancer Applications |
|---|---|---|
| Circulating Tumor DNA (ctDNA) | Tumor-derived fragmented DNA in circulation [31] [32] | Detection of actionable mutations; monitoring treatment response; identifying resistance mechanisms [31] [32] |
| Circulating Tumor Cells (CTCs) | Intact tumor cells shed into bloodstream [31] [32] | Prognostic stratification; monitoring metastatic potential; understanding tumor heterogeneity [31] [32] |
| Tumor Extracellular Vesicles (EVs) | Membrane-bound vesicles containing proteins, nucleic acids [31] | Analyzing proteomic and genomic signatures; monitoring tumor progression and response [31] |
| Circulating Cell-Free RNA (cfRNA) | RNA molecules including miRNAs, lncRNAs [31] | Early detection; monitoring tumor progression; understanding regulatory mechanisms [31] |
| Tumor-Educated Platelets (TEPs) | Platelets containing tumor-derived biomolecules [31] | Detecting cancer biomarkers; monitoring progression and metastasis [31] |
Experimental Protocols for Biomarker Analysis in Liquid Biopsy
Circulating Tumor DNA (ctDNA) Isolation and Analysis
The isolation and analysis of ctDNA requires meticulous pre-analytical procedures to ensure sample integrity and analytical sensitivity. Blood samples should be collected in specialized tubes containing stabilizers to prevent white blood cell lysis and contamination of ctDNA with genomic DNA from hematopoietic cells. Within 4-6 hours of collection, plasma must be separated through a two-step centrifugation protocol: initial centrifugation at 1,600-2,000 × g for 10-20 minutes at 4°C to obtain plasma, followed by a second centrifugation at 16,000 × g for 10 minutes to remove remaining cellular debris [31]. The resulting plasma can be stored at -80°C until DNA extraction.
ctDNA extraction should be performed using commercially available kits optimized for cell-free DNA, with quality control measures including fluorometric quantification and fragment size analysis to confirm the characteristic 166 bp nucleosomal pattern. For mutation detection, digital PCR (dPCR) or next-generation sequencing (NGS) panels provide the required sensitivity for detecting low-frequency variants in a background of wild-type DNA [31]. For dPCR, the protocol involves designing specific probes for target mutations, partitioning the sample into thousands of individual reactions, amplification, and fluorescence reading to absolutely quantify mutant allele frequency. For NGS-based approaches, unique molecular identifiers (UMIs) should be incorporated during library preparation to correct for amplification biases and sequencing errors, enabling accurate quantification of variant allele frequencies down to 0.1% in some applications [31].
Circulating Tumor Cell (CTC) Enrichment and Characterization
CTC analysis begins with blood collection in preservative tubes to maintain cell viability, with processing recommended within 24-48 hours. CTC enrichment typically employs either label-dependent approaches using antibodies against epithelial cell adhesion molecule (EpCAM) or other epithelial markers, or label-free methods leveraging physical properties such as size, density, or electrical characteristics [31]. Immunomagnetic separation provides high purity but may miss epithelial-to-mesenchymal transitioned (EMT) CTCs that have downregulated epithelial markers, while size-based filtration methods preserve all CTC subtypes but may have lower purity.
Following enrichment, CTC characterization typically involves immunocytochemical staining for positive markers (e.g., cytokeratins) and negative markers (e.g., CD45) to confirm epithelial origin and exclude hematopoietic cells. Molecular analysis of CTCs may include whole transcriptome analysis, targeted RNA sequencing, or single-cell DNA sequencing to explore heterogeneity and identify therapeutic targets [31]. For functional characterization, ex vivo culture of CTCs or patient-derived xenograft models can provide insights into drug sensitivity and resistance mechanisms, though these approaches are technically challenging and low-throughput.
Extracellular Vesicle (EV) Isolation and Cargo Analysis
EV isolation requires careful consideration of the trade-offs between yield, purity, and functionality. Differential ultracentrifugation remains the most widely used method, involving sequential centrifugation steps to remove cells and debris (300 × g for 10 minutes, 2,000 × g for 20 minutes, 10,000 × g for 30 minutes) followed by high-speed centrifugation (100,000 × g for 70-120 minutes) to pellet EVs [31]. Alternative approaches include density gradient centrifugation, size-exclusion chromatography, polymer-based precipitation, and immunoaffinity capture using antibodies against EV surface markers (e.g., CD9, CD63, CD81).
For cargo analysis, EV membranes must be lysed using detergent-based buffers or repeated freeze-thaw cycles to release internal contents. RNA extraction should be performed using phenol-chloroform methods or commercial kits specifically validated for small RNA species, with quality assessment using bioanalyzer systems to confirm the presence of small RNAs including miRNAs. Protein analysis typically involves mass spectrometry-based proteomics or immunoassays for specific protein biomarkers. Emerging techniques for direct EV analysis without lysis include nanoparticle tracking analysis for size and concentration determination, and single-EV analysis methods using advanced flow cytometry or imaging techniques [31].
Research Reagent Solutions for Biomarker Studies
Table 3: Essential Research Reagents for Liquid Biopsy Workflows
| Reagent Category | Specific Examples | Function in Workflow |
|---|---|---|
| Blood Collection Tubes | Cell-free DNA BCT tubes, EDTA tubes with preservatives | Stabilize blood samples; prevent contamination from hematopoietic cell genomic DNA [31] |
| Nucleic Acid Extraction Kits | QIAamp Circulating Nucleic Acid Kit, Maxwell RSC ccfDNA Plasma Kit | Isolate high-quality ctDNA/cfRNA; maintain fragment integrity for downstream analysis [31] |
| Library Preparation Kits | AVENIO ctDNA Library Preparation Kits, QIAseq Targeted DNA Panels | Prepare sequencing libraries with unique molecular identifiers (UMIs) for NGS applications [31] |
| PCR Reagents | ddPCR Supermix for Probes, TaqMan Genotyping Master Mix | Enable sensitive mutation detection and absolute quantification in digital PCR applications [31] |
| CTC Enrichment Kits | CellSearch CTC Kits, MACS MicroBead Kit | Islect and enumerate circulating tumor cells from peripheral blood [31] |
| EV Isolation Reagents | ExoQuick-TC, Total Exosome Isolation Reagent | Precipitate or capture extracellular vesicles from plasma and other biofluids [31] |
| Immunoassay Reagents | ELISA kits, Luminex multiplex panels | Quantify protein biomarkers in serum/plasma; enable high-throughput screening [31] |
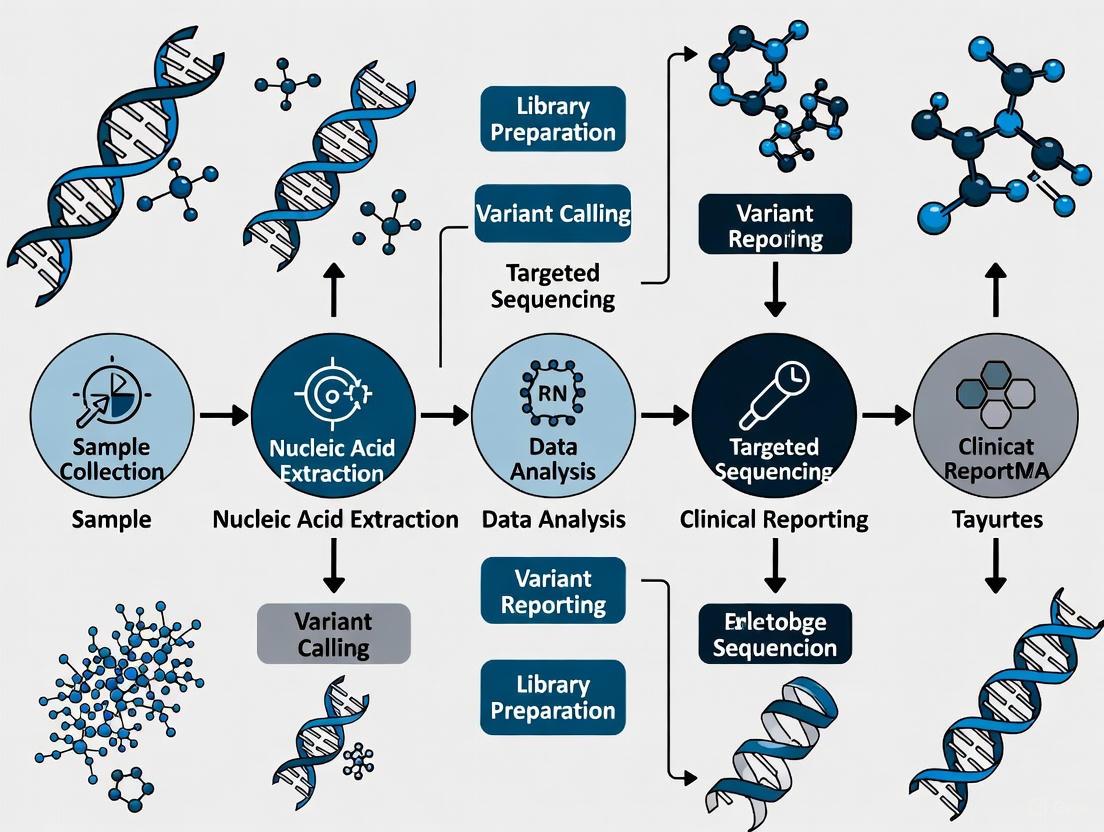
Workflow Visualization
Liquid Biopsy Workflow Overview
Biomarker Relationships and Technologies
Advanced Methodologies and Clinical Applications in Liquid Biopsy Workflows
Liquid biopsy has emerged as a transformative tool in cancer research and management, offering a minimally invasive means to repeatedly sample tumor-derived genetic material. The analytical phase of liquid biopsy, particularly the analysis of cell-free DNA (cfDNA), has advanced significantly with the advent of highly sensitive molecular techniques. However, the pre-analytical phase—encompassing blood collection, processing, and cfDNA extraction—remains a critical source of variability that can profoundly impact downstream analytical results and their clinical interpretation. In fact, studies indicate that pre-analytical errors contribute to 60-70% of total laboratory errors [33]. This application note provides a detailed protocol and critical considerations for standardizing the pre-analytical workflow for cfDNA-based cancer monitoring research, ensuring reliable and reproducible results.
Blood Collection and Initial Processing
Blood Collection Tube Selection
The choice of blood collection tube is the first critical decision point in the liquid biopsy workflow, as it determines sample stability, allowable processing timeframes, and potential sources of contamination. The table below compares the performance characteristics of commonly used tubes based on recent studies:
Table 1: Performance Comparison of Blood Collection Tubes for Liquid Biopsy
| Tube Type | Additive/Mechanism | Recommended Time to Plasma Processing | Relative cfDNA Yield (0h) | Key Advantages | Key Limitations |
|---|---|---|---|---|---|
| K2EDTA/K3EDTA | Chelating agent | <1-2 hours [34] [35] | 2.41 ng/mL [34] | Standard tube, multi-purpose, no special handling required | Short stability window, risk of genomic DNA contamination from leukocyte lysis |
| Streck | Chemical crosslinking [36] [34] | Up to 7 days at RT [34] | 2.74 ng/mL [34] | Excellent stability at room temperature, prevents leukocyte lysis | Lower plasma volume recovery (mean=3.48mL) [34], specific centrifugation conditions |
| PAXgene | Biological apoptosis prevention [36] [34] | Up to 14 days at RT [36] | 1.66 ng/mL [34] | Good stability, compatible with DNA and RNA preservation | 49.4% increase in cfDNA yield over 7 days [34] |
| Norgen | Osmotic cell stabilization [36] [34] | Up to 30 days at RT [36] | 0.76 ng/mL [34] | Longest stability, compatible with DNA and RNA preservation | Lowest initial cfDNA yield, requires single centrifugation only [34] |
Plasma Processing Protocol
Proper plasma processing is essential to prevent contamination by genomic DNA from lysed leukocytes. The following protocol is optimized for cfDNA preservation and purity:
Materials
- Selected blood collection tubes (from Table 1)
- Centrifuge with swing-out rotor and temperature control
- Sterile pipettes and aerosol-resistant tips
- Polypropylene tubes for plasma storage (e.g., Eppendorf Safe-Lock tubes)
- -80°C freezer for plasma storage
Method
Initial Centrifugation:
Plasma Transfer:
- Carefully transfer the upper plasma layer to a fresh polypropylene tube using a sterile pipette, avoiding the buffy coat and platelet layer.
- Leave approximately 0.5 cm of plasma above the buffy coat to prevent cellular contamination.
Secondary Centrifugation:
- Centrifuge the transferred plasma at 6000 × g for 10 minutes at 20°C [35].
- This step removes residual cells and platelets that could contaminate the cfDNA with genomic DNA.
Plasma Aliquoting and Storage:
- Transfer the supernatant to fresh tubes in small aliquots (recommended: 0.5-1 mL) to avoid repeated freeze-thaw cycles.
- Store plasma at -80°C within 30 minutes of the second centrifugation [35].
Critical Considerations
- Processing Time: K2EDTA tubes show significant increases in cfDNA concentration over time (7.39 ng/mL at 48h, 68.19 ng/mL at 168h) due to leukocyte lysis, emphasizing the need for rapid processing [34].
- Centrifugation Conditions: The number of centrifugation steps affects yield. For K2EDTA, Norgen, and PAXgene tubes, a single centrifugation yields higher cfDNA concentrations than double centrifugation [34].
- Temperature Control: Maintain consistent temperature during processing (typically 20°C) to prevent cell lysis.
cfDNA Extraction Methods
Comparison of Extraction Technologies
Multiple cfDNA extraction technologies are available, each with different binding chemistries, efficiencies, and size selectivities. The recovery efficiency and characteristics of these methods directly impact downstream analysis sensitivity, particularly for detecting low-frequency mutations in circulating tumor DNA (ctDNA).
Table 2: Performance Comparison of cfDNA Extraction Methods
| Extraction Method | Technology | Relative Yield | Processing Time | Size Selectivity | Automation Compatibility |
|---|---|---|---|---|---|
| QIAamp Circulating Nucleic Acid Kit (QiaS) | Spin column (silica membrane) [35] | Highest yield [35] | Standard (∼1-2 hours) | Broad range (50-800 bp) [35] | Limited (vacuum manifold) [35] |
| PHASIFY MAX | Liquid-phase (aqueous two-phase system) [37] | 60% increase vs. QiaS [37] | Standard | Enhanced recovery of small fragments [37] | No |
| PHASIFY ENRICH | Liquid-phase with size selection [37] | 35% decrease vs. QiaS [37] | Standard | Enriches fragments <500 bp [37] | No |
| Magnetic Bead with DMS | Magnetic beads with homobifunctional crosslinker [38] | 56% increase vs. QiaS [38] | Rapid (10 minutes) [38] | Preferentially binds small DNA fragments | Yes |
| MagMAX Cell-Free DNA Isolation Kit (TFiM) | Magnetic beads (silica) [35] | Moderate yield [35] | Standard | Broad range | Yes [35] |
| NucleoSpin Plasma XS (MNaS) | Spin column [35] | Lowest yield [35] | Standard | Small input volume (240μL) | No |
Detailed cfDNA Extraction Protocol: QIAamp Circulating Nucleic Acid Kit
This protocol represents the current gold standard for manual cfDNA extraction, providing high yield and reproducibility [35]. The procedure is optimized for 1-4 mL of plasma as starting material.
Materials
- QIAamp Circulating Nucleic Acid Kit (Qiagen)
- Water bath or heating block (set to 60°C)
- Microcentrifuge
- Vacuum manifold (QIAvac 24 Plus) with vacuum pump
- Proteinase K
- Carrier RNA
- Ethanol (96-100%)
- Buffer ACL
- Buffer ACB
- Wash Buffer AW1
- Wash Buffer AW2
Method
Lysis Procedure:
- Thaw frozen plasma samples at room temperature or in a refrigerator overnight.
- Prepare lysis buffer (Buffer ACL) with 1 μg carrier RNA per 1 mL plasma to enhance recovery of low-abundance cfDNA.
- In a 15 mL conical tube, add plasma (1-4 mL), then add Proteinase K solution (volume = 1/10 plasma volume), and Buffer ACL (volume = 4/5 plasma volume).
- Vortex for 30 seconds to mix thoroughly.
- Incubate at 60°C for 30 minutes to digest proteins and nucleases.
Binding Conditions Setup:
- Add Binding Buffer ACB (volume = 9/5 plasma volume) to the lysate.
- Vortex for 30 seconds and incubate on ice for 5 minutes to optimize DNA binding conditions.
- The final mixture volume will range from 1.85 mL (for 0.5 mL plasma) to 3.70 mL (for 1 mL plasma).
cfDNA Binding to Silica Membrane:
- Assemble the spin column with extender on the QIAvac 24 Plus manifold.
- Apply the mixture to the spin column and apply vacuum until all liquid passes through the membrane.
- This step binds cfDNA to the silica membrane while contaminants pass through.
Wash Steps:
- Add 600 μL of Wash Buffer AW1 to the column and apply vacuum.
- Add 600 μL of Wash Buffer AW2 to the column and apply vacuum.
- Add 250 μL of ethanol (96-100%) to the column and apply vacuum to dry the membrane completely.
Elution:
- Place the column in a clean 2 mL collection tube and centrifuge at full speed (≥20,000 × g) for 3 minutes to remove residual ethanol.
- Transfer the column to a new 1.5 mL microcentrifuge tube.
- Apply 20-80 μL of Buffer AVE (elution buffer) to the center of the membrane.
- Incubate at room temperature for 3-5 minutes.
- Centrifuge at full speed for 1 minute to elute the cfDNA.
Storage:
- Store isolated cfDNA at -20°C in DNA LoBind tubes to prevent adsorption and degradation.
Protocol Modifications for Other Methods
For PHASIFY Methods [37]:
- The PHASIFY MAX method utilizes a series of aqueous two-phase systems (ATPSs) that partition cfDNA into specific phases based on optimized electrostatic, hydrophilic/hydrophobic, and excluded-volume interactions.
- The PHASIFY ENRICH method incorporates an additional size-selection step using ATPS components that preferentially precipitate large molecular weight DNA (>500 bp), enriching for smaller cfDNA fragments.
For Magnetic Bead with DMS Method [38]:
- This rapid method utilizes dimethyl suberimidate (DMS) as a homobifunctional crosslinker that binds cfDNA through covalent or electrostatic bonding.
- The DMS-DNA complexes are then captured by amine-conjugated magnetic beads, washed, and eluted under alkaline conditions (pH 10.3) to break the crosslinking.
Quality Control and Normalization
Assessment of Extraction Efficiency and Purity
Robust quality control is essential to ensure cfDNA quality and suitability for downstream applications such as droplet digital PCR (ddPCR) or next-generation sequencing (NGS).
Quantification Methods
- Fluorometric Methods (Qubit): Provide sensitive, DNA-specific quantification but no size information [35].
- qPCR Assays: Offer high sensitivity and the ability to assess fragment size distribution through amplification of targets of different lengths [34].
- Bioanalyzer/TapeStation: Provide fragment size distribution profiles, confirming the expected nucleosomal pattern (peaks at ∼166 bp) and assessing genomic DNA contamination [36] [35].
Assessment of Contaminating Genomic DNA
- Long vs. Short qPCR Assays: Compare amplification of short targets (∼60-80 bp, representative of cfDNA) versus long targets (>200 bp, indicative of high molecular weight genomic DNA) [34].
- Capillary Electrophoresis: Visualize the size distribution profile; a prominent high molecular weight fraction indicates genomic DNA contamination [34].
Spike-in Controls for Normalization
- Synthetic spike-in controls, such as the 180 bp CEREBIS (Construct to Evaluate the Recovery Efficiency of cfDNA extraction and Bisulphite modification) fragment, can be added to samples before extraction to determine and normalize for extraction efficiency [39].
- Studies show extraction efficiencies are method-specific: 84.1% (± 8.17) for the QIAamp kit in plasma, 58.7% (± 11.1) for the Zymo Urine Kit, and 30.2% (± 13.2) for the Q Sepharose protocol [39].
Technical and Biological Variability
Understanding sources of variability is crucial for experimental design and data interpretation:
- Technical Variability: The largest proportion of technical variance in plasma cfDNA extraction is attributed to intra-extraction variability and ddPCR measurement triplicates [39].
- Biological Variability: In both plasma and urine, inter-individual variability comprises the largest proportion of total variance, substantially exceeding technical variability [39].
- Normalization Strategies: For urinary cfDNA, creatinine normalization reduces variability, while CEREBIS-based extraction efficiency normalization shows benefit primarily when comparing different extraction methods [39].
Workflow Visualization
Liquid Biopsy Pre-analytical Workflow: This diagram illustrates the comprehensive workflow from blood collection to downstream analysis, highlighting critical decision points at each stage.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential Research Reagents and Materials for cfDNA Analysis
| Category | Item | Function/Application | Key Considerations |
|---|---|---|---|
| Blood Collection | K2EDTA/K3EDTA Tubes | Standard blood collection for immediate processing | Process within 1-2 hours to prevent gDNA contamination [33] [35] |
| Streck Cell-Free DNA BCT | Blood collection with preservative for delayed processing | Enables room temperature storage for up to 7 days [36] [34] | |
| PAXgene Blood ccfDNA Tubes | Blood collection with apoptosis inhibition | Compatible with DNA and RNA analysis, 14-day stability [36] | |
| cfDNA Extraction | QIAamp Circulating Nucleic Acid Kit | Silica-membrane based cfDNA extraction | Highest yield, spin-column technology [35] |
| PHASIFY MAX/ENRICH Kits | Liquid-phase extraction using aqueous two-phase systems | Enhanced recovery of small fragments, size-selection capability [37] | |
| MagMAX Cell-Free DNA Isolation Kit | Magnetic bead-based extraction | Amenable to automation, moderate yield [35] | |
| DMS (Dimethyl Suberimidate) | Homobifunctional crosslinker for bead-based extraction | Rapid processing (10 minutes), 56% higher efficiency than QIAamp [38] | |
| Quality Control | Qubit Fluorometer with dsDNA HS Assay | Fluorometric quantification of cfDNA | DNA-specific quantification, sensitive to 10 pg/μL [35] |
| Bioanalyzer/TapeStation with High-Sensitivity DNA Kit | Fragment size distribution analysis | Confirms nucleosomal pattern, detects gDNA contamination [36] [35] | |
| CEREBIS Spike-in Control | Synthetic DNA fragment for extraction efficiency normalization | 180 bp fragment mimics mononucleosomal cfDNA [39] | |
| Downstream Analysis | Droplet Digital PCR (ddPCR) | Absolute quantification of rare mutations | High sensitivity for low-frequency variants [37] |
| Next-Generation Sequencing (NGS) | Comprehensive mutation profiling | Requires high-quality, pure cfDNA with minimal gDNA contamination [40] | |
| PROTAC EED degrader-1 | PROTAC EED degrader-1, MF:C55H60FN11O8S, MW:1054.2 g/mol | Chemical Reagent | Bench Chemicals |
| ATX inhibitor 5 | ATX inhibitor 5, MF:C22H18ClF3N6O, MW:474.9 g/mol | Chemical Reagent | Bench Chemicals |
The pre-analytical phase of liquid biopsy testing represents a critical determinant of success in cancer monitoring research. Standardization of blood collection, processing, and cfDNA extraction protocols is essential to minimize technical variability and ensure reproducible, reliable results. Key considerations include:
- Tube Selection: Match collection tubes to logistical constraints, prioritizing preservative tubes when immediate processing is not feasible.
- Processing Conditions: Adhere to standardized centrifugation protocols and processing timelines to prevent genomic DNA contamination.
- Extraction Method Selection: Choose extraction methods based on required yield, fragment size selectivity, and downstream application needs.
- Quality Control: Implement rigorous QC measures including spike-in controls for normalization and multiple assessment methods to verify cfDNA quality and quantity.
By adhering to these detailed protocols and considerations, researchers can significantly reduce pre-analytical variability, enhancing the sensitivity and reliability of liquid biopsy applications in cancer research and drug development.
Comprehensive Genomic Profiling (CGP) Assays for Actionable Variant Detection
Comprehensive Genomic Profiling (CGP) represents a next-generation sequencing (NGS) approach that uses a single assay to assess hundreds of cancer-related genes simultaneously. In the context of liquid biopsy workflows, CGP enables the minimally invasive detection of actionable variants from circulating tumor DNA (ctDNA) and other blood-based biomarkers, providing crucial insights for cancer monitoring research [41] [42]. Liquid biopsy focuses on the analysis of circulating tumor biomarkers from bodily fluids such as blood, offering a dynamic window into tumor heterogeneity and evolution without the need for invasive tissue sampling [43]. This approach is particularly valuable for tracking cancer progression, monitoring therapeutic responses, and detecting minimal residual disease (MRD) through serial sampling [42] [43].
Table 1: Key Biomarkers in Liquid Biopsy for Comprehensive Genomic Profiling
| Biomarker | Description | Role in CGP | Approximate Abundance in Blood |
|---|---|---|---|
| Circulating Tumor DNA (ctDNA) | Fragmented DNA released from tumor cells into circulation | Detection of somatic mutations, CNVs, fusions; monitoring tumor burden | 0.1-1.0% of total cell-free DNA [42] |
| Circulating Tumor Cells (CTCs) | Intact tumor cells shed into bloodstream | Analysis of whole genomes, transcriptomes; functional studies | ~1 CTC per 1 million leukocytes [42] |
| Exosomes/Extracellular Vesicles | Membrane-bound vesicles carrying nucleic acids, proteins | Analysis of RNA, DNA, and protein biomarkers; tumor microenvironment insight | Varies; generally more abundant than CTCs [43] |
Current CGP Assay Platforms and Technologies
Multiple commercial and laboratory-developed CGP platforms now enable comprehensive variant detection from liquid biopsy samples. These systems provide automated, end-to-end workflows from sample preparation to data analysis, making CGP accessible for clinical research settings [41] [44] [45].
Table 2: Comparison of Comprehensive Genomic Profiling Platforms Compatible with Liquid Biopsy
| Platform/Assay | Technology Basis | Genes Covered | Variant Types Detected | Turnaround Time | Key Features |
|---|---|---|---|---|---|
| TruSight Oncology Comprehensive (Illumina) | NGS-based DNA and RNA sequencing | 500+ genes [41] | SNVs, indels, CNVs, fusions [41] | Varies by platform | FDA-approved; pan-cancer claims; automated workflow [41] |
| Oncomine Comprehensive Assay Plus (Thermo Fisher) | Ion AmpliSeq technology | 517 genes [45] | SNVs, indels, CNVs, fusions, TMB, MSI, HRD [45] | 1-3 days [45] | Genomic signatures; low input requirements (20-30 ng DNA) [45] |
| MSK-IMPACT/MSK-ACCESS (via Complete Genomics) | DNBSEQ technology | Varies by application | SNVs, indels, CNVs, fusions | Varies by platform | Integrated with SOPHiA DDM analytics; validated for decentralized labs [44] |
Experimental Protocols for Liquid Biopsy CGP Workflow
Sample Collection and Processing Protocol
Principle: Proper pre-analytical sample handling is critical for successful liquid biopsy CGP due to the low abundance of ctDNA in circulation [42] [43].
Materials:
- Streck Cell-Free DNA Blood Collection Tubes or equivalent
- Centrifuge capable of 1600-3000 × g
- DNA extraction kit optimized for cell-free DNA
- Qubit fluorometer or equivalent for DNA quantification
- Agarose gel or Bioanalyzer for quality control
Procedure:
- Blood Collection: Collect 10-20 mL peripheral blood into cell-free DNA stabilization tubes. Invert gently 8-10 times for mixing.
- Plasma Separation: Centrifuge at 1600-3000 × g for 10-20 minutes at 4°C within 2-6 hours of collection.
- Secondary Centrifugation: Transfer supernatant plasma to fresh tubes. Centrifuge at 16,000 × g for 10 minutes to remove residual cells.
- Plasma Storage: Aliquot cleared plasma and store at -80°C if not processing immediately.
- cfDNA Extraction: Extract cell-free DNA using silica membrane or bead-based methods according to manufacturer protocols.
- DNA Quantification and QC: Quantify DNA using fluorometric methods. Assess fragment size distribution (expected peak ~160-170 bp).
Technical Notes:
- Process samples within 6 hours of draw for optimal cfDNA preservation
- Minimum input requirements typically 20-50 ng total cfDNA for CGP assays [45]
- Avoid repeated freeze-thaw cycles of plasma samples
Library Preparation and Sequencing Protocol
Principle: CGP assays employ hybrid capture or amplicon-based approaches to enrich for hundreds of cancer-related genes prior to sequencing [41] [45].
Materials:
- Library preparation kit specific to CGP platform (e.g., TruSight Oncology, Oncomine)
- Magnetic bead-based purification system
- Platform-specific sequencing instruments (Illumina, Ion Torrent, DNBSEQ)
- Indexing primers for sample multiplexing
Procedure:
- Library Preparation: Fragment DNA (if required) and add platform-specific adapters following manufacturer protocols.
- Target Enrichment: Hybridize libraries with biotinylated probes targeting the CGP gene panel. Use streptavidin beads to capture target regions.
- Library Amplification: Perform PCR amplification of captured libraries using high-fidelity polymerases.
- Library QC: Assess library concentration and size distribution using fluorometry and fragment analyzers.
- Normalization and Pooling: Normalize libraries by concentration and pool for multiplexed sequencing.
- Sequencing: Load onto appropriate sequencing platform to achieve minimum 500x average coverage with >95% of bases at ≥100x coverage.
Technical Notes:
- Include positive control samples with known variant allele frequencies for QC
- Incorporate unique molecular identifiers (UMIs) to correct for PCR duplicates and sequencing errors
- Adjust sequencing depth based on expected ctDNA fraction (typically 0.1-1%)
Bioinformatic Analysis and Interpretation
Data Processing Workflow: Raw sequencing data undergoes a multi-step bioinformatic pipeline to identify actionable variants with clinical significance.
Variant Interpretation Logic: Identified variants undergo a structured classification process to determine clinical actionability and support research decisions.
Research Reagent Solutions for CGP Workflows
Table 3: Essential Research Reagents and Materials for Liquid Biopsy CGP
| Reagent/Material | Function | Example Products | Application Notes |
|---|---|---|---|
| cfDNA Preservation Tubes | Stabilizes nucleated blood cells and prevents cfDNA release | Streck Cell-Free DNA BCT, PAXgene Blood cDNA Tube | Critical for sample integrity; enables sample transport |
| Nucleic Acid Extraction Kits | Isolation of high-quality cfDNA from plasma | QIAamp Circulating Nucleic Acid Kit, MagMAX Cell-Free DNA Isolation Kit | Optimized for low-abundance targets; minimize contamination |
| Library Preparation Kits | Fragment end-repair, adapter ligation, and target enrichment | TruSight Oncology Library Prep, Oncomine cfDNA Assay | Platform-specific; include UMI technology |
| Target Capture Panels | Hybridization baits for specific gene enrichment | IDT xGen Pan-Cancer Panel, Illumina TSO Comprehensive | Determine genomic coverage; 500+ genes for CGP |
| Sequenceing Platforms | High-throughput DNA sequencing | Illumina NovaSeq, DNBSEQ-G400, Ion GeneStudio S5 | Different throughput options for various lab scales |
Applications in Cancer Monitoring Research
Liquid biopsy CGP enables multiple research applications that leverage its minimally invasive nature and comprehensive genomic coverage. Key applications include:
Treatment Response Monitoring: Serial liquid biopsies can track changes in variant allele frequencies of actionable mutations, providing early indicators of treatment efficacy or emergence of resistance [42] [43]. Research studies have demonstrated that decreasing ctDNA levels often precede radiographic evidence of response, while rising levels may indicate emerging resistance.
Minimal Residual Disease (MRD) Detection: The high sensitivity of CGP assays enables detection of ctDNA at very low variant allele frequencies (0.01% or lower), allowing researchers to identify MRD following curative-intent treatment [44] [43]. Recent studies have shown that ctDNA-based MRD detection can predict recurrence months before clinical manifestation.
Tumor Heterogeneity Assessment: By capturing the full spectrum of genomic alterations present in different tumor subclones, liquid biopsy CGP provides insights into tumor evolution and heterogeneity that may be missed by single-site tissue biopsies [42] [43]. This comprehensive profiling supports research into resistance mechanisms and clonal dynamics.
Clinical Trial Enrichment: CGP facilitates identification of rare actionable variants across cancer types, enabling precision oncology trial designs that enroll patients based on molecular alterations rather than tumor histology [41]. This approach accelerates targeted therapy development and improves trial efficiency.
Quality Control and Validation
Robust quality control measures are essential throughout the liquid biopsy CGP workflow due to the technical challenges of analyzing low-abundance ctDNA.
Pre-analytical QC:
- Plasma volume and processing time tracking
- cfDNA yield quantification (minimum 20 ng recommended)
- Fragment size analysis (peak ~160-170 bp expected)
Analytical QC:
- Sequencing metrics: average coverage (>500x), uniformity (>95% bases at ≥100x)
- Control sample performance (positive and negative controls)
- Limit of detection validation for low-frequency variants
Post-analytical QC:
- Variant call reproducibility across technical replicates
- Validation of potentially actionable findings with orthogonal methods when possible
- Database annotation consistency and version control
Implementing these comprehensive protocols and quality measures ensures reliable detection of actionable variants in liquid biopsy samples, supporting robust cancer monitoring research and advancing the field of precision oncology.
DNA Methylation Analysis and Fragmentomics for Early Detection
Liquid biopsy, the analysis of tumor-derived material in bodily fluids, represents a paradigm shift in non-invasive cancer monitoring [20]. Two particularly promising analytical domains within this field are DNA methylation analysis and cfDNA fragmentomics. DNA methylation involves the addition of a methyl group to cytosine, primarily at CpG dinucleotides, and in cancer, promoter regions of tumor suppressor genes often become hypermethylated, leading to gene silencing [20] [22]. These alterations are stable, occur early in tumorigenesis, and provide a specific cancer signal [20] [46]. Conversely, cfDNA fragmentomics entails the genome-wide analysis of fragmentation patterns of cell-free DNA, including features like fragment length distributions, end motifs, and nucleosome footprints, which are perturbed in cancer [47]. When integrated, these approaches offer a powerful, multi-modal framework for the early detection of cancer, assessment of minimal residual disease, and monitoring of treatment response, which is the central focus of this research thesis [20] [47].
The following tables summarize key performance metrics from recent studies utilizing DNA methylation and fragmentomics for early cancer detection.
Table 1: Performance of Selected DNA Methylation Biomarkers in Early Cancer Detection
| Cancer Type | Methylation Biomarker(s) | Sample Type | Detection Method | Sensitivity / Specificity / AUC | Citation |
|---|---|---|---|---|---|
| Breast Cancer | Panel of 15 ctDNA biomarkers | Blood (Plasma) | Whole-genome bisulfite sequencing | AUC: 0.971 | [22] |
| Colorectal Cancer | SDC2, SFRP2, SEPT9 | Feces, Blood | Real-time PCR with fluorescent probe | Sensitivity: 86.4%, Specificity: 90.7% | [22] |
| Breast Cancer | TRDJ3, PLXNA4, KLRD1, KLRK1 | PBMC, Tissue, Blood | Pyrosequencing, Targeted bisulfite sequencing | Sensitivity: 93.2%, Specificity: 90.4% | [22] |
| Lung Cancer | SHOX2, RASSF1A | Blood, Bronchoalveolar lavage fluid | Methylight, NGS | Sensitivity: 67-73%, Specificity: 82-90% | [22] [48] |
| Esophageal Squamous Cell Carcinoma | Panel of 12 methylated CpG sites | Tissue, Blood | Microarray, Real-time PCR | AUC: 0.966 | [22] |
Table 2: Performance of cfDNA Fragmentomics in Early Cancer Detection
| Cancer Type | Fragmentomic Features Analyzed | Sample Type | Detection Method | Sensitivity / Specificity / AUC | Citation |
|---|---|---|---|---|---|
| Renal Cell Carcinoma (All stages) | Copy number variation, Fragment size ratio, Nucleosome footprint | Blood (Plasma) | Low-pass whole genome sequencing (5X coverage) + Machine Learning | AUC: 0.96, Sensitivity: 90.5%, Specificity: 93.8% | [49] |
| Renal Cell Carcinoma (Stage I) | Copy number variation, Fragment size ratio, Nucleosome footprint | Blood (Plasma) | Low-pass whole genome sequencing (5X coverage) + Machine Learning | Sensitivity: 87.8% | [49] |
| Multiple Cancers | Fragment size, End motifs, Nucleosome footprints, Copy number variations | Blood (Plasma) | Low-pass WGBS, Multidimensional analysis | High performance for early detection (Specific metrics under review) | [47] [46] |
DNA Methylation Analysis: Experimental Protocols
Protocol: Bisulfite Sequencing for Genome-Wide Methylation Profiling
Bisulfite sequencing is the gold standard for single-base resolution DNA methylation analysis. This protocol outlines the steps for Whole-Genome Bisulfite Sequencing (WGBS) and the subsequent computational analysis [50].
Principle: Treatment of DNA with bisulfite converts unmethylated cytosines to uracils (which are read as thymines in sequencing), while methylated cytosines remain unchanged. The sequencing output allows for the quantitative comparison of methylation levels at each cytosine in the genome [22] [50].
Workflow Overview:
Step-by-Step Methodology:
DNA Extraction and Quality Control:
- Extract high-quality DNA from your source (e.g., plasma cfDNA, tissue, PBMCs). The required input can be as low as 1 ng for optimized cfDNA protocols [46].
- Quantify DNA using fluorometric methods (e.g., Qubit) and assess integrity (e.g., Bioanalyzer/TapeStation).
Bisulfite Conversion:
- Use a commercial bisulfite conversion kit (e.g., EZ DNA Methylation-Lightning Kit, Qiagen Epitect Bisulfite Kit).
- Procedure: Dilute DNA in a volume of ≤ 20 µL. Add the bisulfite conversion reagent and run the thermocycler program as per kit instructions (typically involving incubation at 95-98°C for denaturation, followed by 50-60°C for several hours for conversion). Desulphonate and elute the converted DNA in a small volume (e.g., 10-20 µL).
Library Preparation for Sequencing:
- Use a library prep kit designed for bisulfite-converted DNA (e.g., Accel-NGS Methyl-Seq DNA Library Kit, Swift Biosciences).
- Procedure: Perform end-repair and adenylation of the converted DNA fragments. Ligate methylated adapters to the DNA fragments. Amplify the library via PCR (5-12 cycles) using indexing primers for sample multiplexing. Clean up the final library using magnetic beads.
Sequencing:
- Quantify the final library by qPCR for accurate molarity.
- Pool multiplexed libraries and sequence on an Illumina platform (NovaSeq, HiSeq, or MiSeq) to a sufficient depth (typically 20-30x coverage for WGBS).
Bioinformatic Analysis [50]:
- Quality Control: Use
FastQCto assess raw read quality. - Read Alignment: Map bisulfite-treated reads to a bisulfite-converted reference genome using aligners like
Bowtie2(in Bismark mode) orBS-Seeker2. - Methylation Calling: Process the aligned BAM files to generate a comprehensive methylation report (e.g., CGmap format), which details the methylation status of each cytosine.
- Differential Methylation Analysis: Identify Differentially Methylated Regions (DMRs) between case and control samples using tools such as
MethylC-analyzerorHOME. - Visualization: Visualize methylation patterns in genomic contexts using tools like the
Integrative Genomics Viewer (IGV).
- Quality Control: Use
Protocol: Targeted Methylation Sequencing
Targeted methylation sequencing focuses on clinically relevant CpG sites, offering a cost-effective and highly sensitive solution for liquid biopsy applications [46].
Principle: Target regions of interest (e.g., known hypermethylated gene promoters in a specific cancer) are enriched from bisulfite-converted DNA using hybrid-capture probes or amplicon-based approaches before sequencing.
Workflow Overview:
Step-by-Step Methodology:
DNA Extraction and Bisulfite Conversion: As described in section 3.1.
Library Preparation and Target Enrichment:
- Prepare sequencing libraries from bisulfite-converted DNA.
- For Hybrid-Capture: Design biotinylated RNA probes complementary to the bisulfite-converted sequence of your target regions. Hybridize the probes to the library, pull down the target-probe complexes using streptavidin beads, and wash away non-specific fragments.
- For Amplicon-Based (e.g., AnchorIRIS, ELSA-seq): Design PCR primers that flank the target CpG sites. Amplify the targets, often using methods that minimize bias and maximize efficiency from fragmented cfDNA [46].
Sequencing and Analysis:
- Sequence the enriched library to a high depth (>1000x) on an Illumina platform.
- Analyze data using alignment and methylation calling pipelines similar to WGBS, but focused on the targeted regions. The high depth allows for highly sensitive detection of low-frequency methylated alleles in a background of normal cfDNA.
cfDNA Fragmentomics Analysis: Experimental Protocols
Protocol: Genome-Wide Fragmentomics Profiling
This protocol outlines the steps for analyzing cancer-related fragmentation patterns from low-pass whole-genome sequencing of cfDNA [47] [49].
Principle: The nucleosome positioning and nuclease activity in tumor cells differ from healthy cells, resulting in distinct cfDNA size distributions, end motifs, and genomic coverage patterns. These can be captured by shallow whole-genome sequencing and machine learning.
Workflow Overview:
Step-by-Step Methodology:
Blood Collection and Plasma Separation:
- Collect blood in cell-stabilizing tubes (e.g., Streck Cell-Free DNA BCT). Process within 6-48 hours by a double-centrifugation protocol (e.g., 1600 × g for 10 min, then 16,000 × g for 10 min) to obtain platelet-poor plasma. Store plasma at -80°C.
cfDNA Extraction:
- Extract cfDNA from 1-10 mL of plasma using a commercial cfDNA extraction kit (e.g., QIAamp Circulating Nucleic Acid Kit, Qiagen). Elute in a small volume (e.g., 20-50 µL) of low-EDTA TE buffer or nuclease-free water.
Library Preparation and Sequencing:
- Prepare sequencing libraries from 5-50 ng of cfDNA using a kit that preserves the native ends and minimizes PCR amplification biases (e.g., KAPA HyperPrep Kit). Use a low number of PCR cycles or PCR-free methods.
- Sequence the library on an Illumina platform to a low coverage of ~5x whole-genome equivalent. This low depth makes the assay cost-effective for clinical application [49].
Bioinformatic Analysis for Fragmentomics:
- Alignment and QC: Align sequenced reads to the human reference genome (e.g., hg38) using standard aligners like BWA-MEM. Remove duplicate reads.
- Feature Extraction:
- Fragment Size Distribution: Calculate the size of every cfDNA fragment from the aligned BAM file. Tumor-derived cfDNA is often enriched in shorter fragments.
- End Motif Analysis: Analyze the nucleotide sequence at the ends of cfDNA fragments (e.g., 4-base end motifs). Cancer patients have distinct end motif preferences compared to healthy individuals [47].
- Nucleosome Footprinting: Analyze the sequencing coverage patterns across the genome. A periodicity of ~167 bp indicates nucleosomal protection, and the loss of this pattern in genomic regions can be a cancer signal.
- Copy Number Variation (CNV): Analyze the read depth across the genome in large bins (e.g., 50-500 kb) to identify somatic copy number alterations, which are common in cancer.
- Machine Learning Integration: Input the extracted fragmentomic features into a trained ensemble or other machine learning model (e.g., stacked classifier) to generate a diagnostic score for cancer detection [49].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Kits for Liquid Biopsy Workflows
| Item Name | Function / Application | Example Vendor/Product |
|---|---|---|
| Cell-Free DNA Collection Tubes | Stabilizes nucleated blood cells for up to 14 days, preventing genomic DNA contamination. | Streck Cell-Free DNA BCT |
| cfDNA Extraction Kit | Isolves short, low-concentration cfDNA from plasma with high efficiency and purity. | QIAamp Circulating Nucleic Acid Kit (Qiagen) |
| Bisulfite Conversion Kit | Chemically converts unmethylated cytosine to uracil for downstream methylation analysis. | EZ DNA Methylation-Lightning Kit (Zymo Research) |
| Methylation-Specific qPCR Assay | Highly sensitive, quantitative detection of specific methylated loci for biomarker validation. | MethyLight, ddPCR assays |
| Methylation Sequencing Library Prep Kit | Prepares NGS libraries from bisulfite-converted DNA, preserving methylation information. | Accel-NGS Methyl-Seq DNA Library Kit (Swift Biosciences) |
| Target Enrichment Probes/Panels | Hybrid-capture or amplicon-based probes to enrich specific genomic regions for targeted sequencing. | Illumina EPIC Array, Custom Panels (IDT, Twist) |
| PCR-Free Library Prep Kit | Prepares NGS libraries without PCR amplification, reducing bias for fragmentomics and WGBS. | KAPA HyperPrep Kit (Roche), Illumina DNA Prep |
| Methylation Analysis Software | Bioinformatics tools for alignment, methylation calling, and DMR discovery from sequencing data. | Bismark, BS-Seeker2, MethylKit |
| Halofuginone Hydrobromide | Halofuginone Hydrobromide | |
| HMN-176 | HMN-176, MF:C20H18N2O4S, MW:382.4 g/mol | Chemical Reagent |
Minimal Residual Disease (MRD) Monitoring and Treatment Response Assessment
Minimal Residual Disease (MRD) refers to the small population of cancer cells that persist in a patient after treatment, typically at levels undetectable by conventional imaging or morphological examination [51]. In hematological malignancies, MRD represents a latent reservoir of disease that can lead to clinical relapse if not properly addressed, while in solid tumors, its detection provides a critical window into subclinical disease status [51] [52]. The ability to detect MRD, even in the absence of clinical symptoms, provides crucial prognostic information that traditional methods may miss, allowing for earlier intervention and more personalized treatment strategies [51]. The clinical utility of MRD monitoring spans multiple domains: it serves as a powerful tool for assessing treatment efficacy, predicting relapse risk, guiding risk-adapted therapy, and evaluating clinical trial endpoints for novel cancer therapeutics [51] [53].
The emergence of liquid biopsy has revolutionized MRD detection by providing a minimally invasive alternative to traditional tissue biopsies [42] [52]. Liquid biopsy enables serial sampling and monitoring of tumor dynamics through the analysis of various biomarkers including circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and other tumor-derived components found in blood and other bodily fluids [42] [54]. This approach captures tumor heterogeneity and provides a comprehensive view of the disease landscape, making it particularly valuable for monitoring treatment response and detecting recurrence earlier than conventional methods [52] [54].
Current MRD Detection Technologies and Performance Specifications
Multiple technological platforms are available for MRD detection, each with distinct advantages, limitations, and optimal applications. The selection of an appropriate method depends on cancer type, available samples, required sensitivity, and clinical context [51].
Table 1: Comparison of Major MRD Detection Methodologies
| Platform | Applicability | Sensitivity | Key Advantages | Major Limitations |
|---|---|---|---|---|
| Next-Generation Sequencing (NGS) | >95% [51] | 10â»Â² to 10â»â¶ [51] | Broad detection of clonal rearrangements & somatic mutations; multiple genes analyzed simultaneously [51] | Complex data analysis; high cost; longer turnaround time; requires professional expertise [51] |
| Flow Cytometry (FCM) | Nearly 100% [51] | 10â»Â³ to 10â»â¶ (increases with panel complexity) [51] | Wide application range; fast turnaround; relatively inexpensive; requires no pre-identified marker [51] | Lack of standardization; changes in immunophenotype; requires fresh cells; demands professional knowledge [51] |
| Quantitative PCR (qPCR) | ~40-50% [51] | 10â»â´ to 10â»â¶ [51] | Highly standardized; widely used; lower cost [51] | Limited to one specific gene or target per assay [51] |
| Droplet Digital PCR (ddPCR) | N/A (Technology not profiled in detail in results) | 0.001% MAF (Mutant Allele Frequency) [52] | Absolute quantification of target DNA; ultra-high sensitivity [52] | Restricted to predefined mutations; limited genomic coverage [52] |
| Fluorescence In Situ Hybridization (FISH) | ~50% [51] | ~10â»Â² [51] | Useful for quantifying specific cytogenetic abnormalities; relatively fast [51] | Labor-intensive; requires pre-existing abnormal karyotype [51] |
For solid tumors, ctDNA-based MRD detection has emerged as the predominant approach due to its sensitivity and specificity [52]. Two principal methodological frameworks are employed: tumor-informed and tumor-naïve (or tumor-agnostic) approaches [52]. Tumor-informed methods require prior sequencing of tumor tissue to identify patient-specific mutations, which are then tracked in plasma using custom assays. This approach offers high specificity and sensitivity, with platforms like Signatera (Natera) and RaDaR (Inivata/NeoGenomics) achieving limits of detection (LoD) as low as 0.001–0.02% mutant allele frequency (MAF) [52]. In contrast, tumor-naïve methods use predefined panels of recurrent cancer-associated genomic or epigenomic alterations and do not require prior tumor sequencing, offering faster turnaround times and broader applicability, though potentially with reduced sensitivity [52]. Key tumor-naïve platforms include Guardant Reveal (Guardant Health) and Oncomine cfDNA Assay (Thermo Fisher Scientific), with LoDs typically ranging from 0.07% to 0.33% MAF [52].
Experimental Protocols for MRD Detection
Robust and standardized pre-analytical and analytical protocols are fundamental to reliable MRD detection. The following sections detail critical protocols for blood collection, plasma processing, and ctDNA-based MRD analysis.
Blood Collection and Plasma Processing Protocol
The pre-analytical phase is critical for sample quality and directly impacts assay performance [36].
Blood Collection: Collect peripheral blood via venipuncture using blood preservation tubes. The choice of tube depends on sample processing logistics [36]:
- K3EDTA Tubes: Process within 1 hour of collection if no preservation tubes are available [36].
- Cell-Free DNA BCT Streck Tubes: Can be stored at room temperature (RT) for up to 14 days before processing. These tubes use a chemical crosslinking approach to stabilize nucleated cells [36].
- PAXgene Blood ccfDNA Tubes: Can be stored at RT for up to 14 days or at 4°C for 28 days. These tubes employ biological apoptosis prevention [36].
- cf-DNA/cf-RNA Preservative Tubes (Norgen): Can be stored at RT for up to 30 days. These tubes rely on osmotic stabilization of nucleated cells [36].
Plasma Separation: Centrifuge blood tubes using a two-step centrifugation protocol [36]:
- First Centrifugation: 500–1900 × g for 10–20 minutes at RT to separate plasma from blood cells.
- Second Centrifugation: Transfer the supernatant (plasma) to a new tube and centrifuge at 16,000 × g for 10 minutes at 4°C to remove any remaining cellular debris. Aliquot the purified plasma into cryovials and store at -80°C until nucleic acid extraction.
ctDNA Extraction and Quality Control Protocol
This protocol describes the parallel isolation of cell-free DNA (cfDNA) and cell-free RNA (cfRNA) from plasma samples, enabling multi-analyte analysis from a single liquid biopsy [36].
Cell-Free Nucleic Acid Isolation: Use commercial kits such as the NucleoSnap and NucleoSpin kits for parallel isolation of cfDNA and cfRNA from 1-4 mL of plasma, following the manufacturer's instructions. Other validated kits can also be used [36].
Quality Control and Quantification:
- Quantification: Use fluorescence-based quantification methods like Qubit for accurate measurement of low-concentration nucleic acids.
- Fragment Analysis: Use Bioanalyzer or TapeStation systems to assess the size distribution and integrity of the extracted cfDNA. The characteristic nucleosomal peak for cfDNA should be approximately 166 base pairs [36].
- Purity Assessment: Calculate cfDNA purity as the ratio of cfDNA (approximately 160 bp peak) to total DNA (50–7000 bp) to quantify genomic DNA contamination, which should be minimized [36].
Tumor-Informed ctDNA MRD Assay Workflow (e.g., for Signatera or RaDaR)
This workflow utilizes prior knowledge of tumor-specific mutations to create a highly sensitive patient-specific assay [52].
Tumor Whole Exome/Genome Sequencing: Isolate DNA from formalin-fixed paraffin-embedded (FFPE) tumor tissue and matched normal blood. Perform whole-exome sequencing (WES) or whole-genome sequencing (WGS) to identify somatic single nucleotide variants (SNVs) and small insertions/deletions (indels) unique to the tumor.
Custom Panel Design: Select 16-50 patient-specific clonal mutations to create a bespoke, multiplex PCR panel for tracking in plasma.
Library Preparation and Sequencing:
- For each plasma timepoint, extract cfDNA as described in Protocol 3.2.
- Use the custom panel to prepare sequencing libraries from the extracted cfDNA. Incorporate Unique Molecular Identifiers (UMIs) to tag individual DNA molecules, enabling error correction and accurate quantification.
- Perform deep sequencing (typically >100,000X coverage) on a high-throughput sequencing platform.
Bioinformatic Analysis and MRD Calling:
- Process raw sequencing data through a bioinformatics pipeline to align sequences, group UMI families, and call variants.
- The sample is classified as MRD-positive if two or more of the patient-specific mutations are detected above a statistically determined background threshold.
- The assay's limit of detection (LoD) is typically between 0.001% and 0.02% tumor fraction (TF) [52].
The following diagram illustrates the key stages of this multi-step protocol:
Tumor-Agnostic ctDNA MRD Assay Workflow (e.g., for Guardant Reveal)
This workflow uses a fixed panel of cancer-associated genomic or epigenomic alterations and does not require prior tumor sequencing [52].
Plasma Collection and cfDNA Extraction: Collect blood in appropriate preservation tubes (see Protocol 3.1). Process within the tube's specified stability window to isolate plasma and extract cfDNA as described in Protocol 3.2.
Library Preparation and Hybrid Capture:
- Prepare sequencing libraries from the extracted cfDNA.
- Use a predefined hybrid-capture panel targeting common mutations (e.g., in genes like TP53, KRAS, APC), methylation patterns, or fragmentomic profiles associated with the cancer type of interest.
- Incorporate UMIs for error correction.
Sequencing and Analysis:
- Perform deep sequencing on a high-throughput platform.
- Analyze data using specialized bioinformatic algorithms to detect aberrant signals (mutations, methylation changes, fragment size patterns) against a background of wild-type cfDNA.
- The sample is classified as MRD-positive if the signal exceeds a predefined threshold derived from healthy controls. The typical LoD for these assays is 0.07% to 0.33% MAF [52].
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful implementation of MRD detection assays requires specific, high-quality reagents and materials throughout the workflow. The following table details key components for ctDNA-based MRD analysis.
Table 2: Essential Research Reagents and Materials for ctDNA MRD Analysis
| Item Category | Specific Examples | Function and Application Notes |
|---|---|---|
| Blood Collection Tubes | Cell-Free DNA BCT (Streck), PAXgene Blood ccfDNA Tubes (Qiagen), cf-DNA/cf-RNA Preservative Tubes (Norgen) [36] | Stabilize nucleated cells to prevent genomic DNA contamination during sample storage and transport. Critical for preserving sample integrity when immediate processing is not possible. |
| Nucleic Acid Extraction Kits | NucleoSnap cfDNA / NucleoSpin cfRNA kits (Macherey-Nagel), QIAamp Circulating Nucleic Acid Kit (Qiagen) [36] | Isolate high-quality, short-fragment cfDNA and cfRNA from plasma samples. Efficiency and purity of extraction directly impact downstream assay sensitivity. |
| Library Prep Kits | Kits for NGS library construction from low-input cfDNA (e.g., from Illumina, Thermo Fisher) | Prepare cfDNA fragments for sequencing, often including adapter ligation and sample indexing. Optimized kits are crucial for working with low-concentration, fragmented cfDNA. |
| Target Enrichment Reagents | Custom multiplex PCR primers (for tumor-informed), Hybrid-capture probes (for tumor-agnostic) [52] | Enrich for patient-specific mutations (tumor-informed) or a panel of cancer-related targets (tumor-agnostic) from the total cfDNA background, enabling deep sequencing. |
| Unique Molecular Identifiers (UMIs) | UMI-containing adapters or primers | Tag individual DNA molecules before PCR amplification to correct for sequencing errors and PCR duplicates, thereby improving quantification accuracy and variant detection. |
| Quantification & QC Kits | Qubit dsDNA HS Assay Kit (Thermo Fisher), Bioanalyzer High Sensitivity DNA Kit (Agilent) | Precisely quantify and qualify nucleic acids at all stages (post-extraction, post-library prep) to ensure adequate material and proper fragment size for sequencing. |
| Bioinformatic Software | Custom pipelines for UMI processing, variant calling, and MRD classification (e.g., provided by platform vendors) [52] | Analyze complex NGS data to distinguish true tumor-derived signals from technical noise and clonal hematopoiesis, ultimately determining MRD status. |
| Tpc2-A1-N | Tpc2-A1-N, MF:C17H9Cl2F3N2O2, MW:401.2 g/mol | Chemical Reagent |
| GPR34 receptor antagonist 2 | GPR34 receptor antagonist 2, MF:C31H26ClNO4, MW:512.0 g/mol | Chemical Reagent |
Data Interpretation and Clinical Integration
Interpreting MRD results requires understanding the analytical performance of the assay used and the clinical context. A key concept is the Limit of Detection (LoD), which is the lowest tumor fraction at which an assay can reliably detect ctDNA. This varies significantly between platforms, from 0.0001% for the most sensitive tumor-informed assays to ~0.1% for some tumor-agnostic tests [52]. The timing of sample collection is also critical for accurate assessment. In the adjuvant setting for solid tumors, the first blood draw is typically taken 2-4 weeks after completion of curative-intent therapy (surgery or chemoradiation) to establish a post-treatment baseline, followed by serial monitoring every 3-6 months for surveillance [52]. In acute myeloid leukemia (AML), MRD assessment is standardly performed after induction and consolidation therapy, with results providing powerful independent prognostic information for relapse risk and survival [53].
The following diagram summarizes the decision-making workflow based on MRD results:
Integrating MRD monitoring into clinical trials and practice is transforming cancer management. MRD status is increasingly used as a surrogate endpoint in clinical trials, accelerating drug development [55]. Furthermore, MRD-directed therapy is becoming a reality, where treatment decisions are guided by MRD results. This includes treatment de-escalation in MRD-negative patients to avoid unnecessary toxicity, and treatment escalation or change in MRD-positive patients to eradicate resistant clones before clinical relapse occurs [52] [53].
Integrating Liquid Biopsy into Clinical Trial Design and Drug Development
Liquid biopsy is revolutionizing oncology drug development by providing a minimally invasive method for detecting and analyzing tumor-derived biomarkers from bodily fluids such as blood [43]. Unlike traditional tissue biopsies, which offer a static snapshot of a single tumor site, liquid biopsy captures the molecular heterogeneity of the entire tumor burden and enables real-time monitoring of cancer evolution throughout treatment [43]. This dynamic capability is particularly valuable in clinical trials, where understanding patient response mechanisms and rapidly identifying effective drug doses can significantly accelerate development timelines.
The foundational biomarkers analyzed in liquid biopsy include circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and extracellular vesicles (EVs) [43] [42]. Among these, ctDNA has emerged as a particularly sensitive tool for tracking tumor dynamics, with a short half-life (minutes to hours) that allows for near real-time assessment of disease status [43] [20]. Technological advances in next-generation sequencing (NGS) and digital PCR have enhanced the sensitivity of these assays, enabling detection of minimal residual disease (MRD) and early resistance mutations that often precede radiographic progression [56] [43].
Key Applications in Drug Development
Dose Selection and Optimization
Liquid biopsy provides a rapid method for determining optimal drug dosing in early-phase clinical trials. The AMPLIFY-201 trial of ELI-002, a therapeutic cancer vaccine candidate, demonstrated how ctDNA monitoring could accelerate dose-finding decisions. Researchers utilized liquid biopsy to more rapidly determine the Phase II dose by observing rapid reduction and clearance of circulating tumor DNA before radiographic changes were evident [56]. This approach enabled the correlation of T-cell immune responses with antitumor effects measured through ctDNA reduction, with 84% of patients showing reduced tumor biomarkers and 24% achieving complete ctDNA clearance [56].
Table 1: Liquid Biopsy Applications in Different Trial Phases
| Trial Phase | Application | Impact | Example |
|---|---|---|---|
| Phase I | Dose escalation decisions | More rapid dose determination than radiographic imaging | AMPLIFY-201: ctDNA clearance observed within weeks of treatment [56] |
| Phase II | Preliminary efficacy assessment | Correlation of mechanism of action with antitumor effect | AMPLIFY-201: 84% of patients showed reduced tumor biomarkers correlating with T-cell responses [56] |
| Phase III | Patient stratification & MRD detection | Smaller, faster trials through enrichment of high-risk populations | ctDNA-positive patients post-surgery have higher relapse risk, enabling smaller trial designs [56] |
| Across Phases | Therapy response monitoring | Early identification of resistance mechanisms | ctDNA dynamics can signal emerging resistance months before clinical progression [43] |
Minimal Residual Disease Monitoring
Detection of minimal residual disease (MRD) represents one of the most clinically established applications of liquid biopsy in drug development. The VICTORI study in colorectal cancer demonstrated that 87% of recurrences were preceded by ctDNA positivity, while no ctDNA-negative patients relapsed [16]. This predictive capability enables drug developers to use MRD status as an enrichment strategy in adjuvant trials, selecting patients at highest risk of recurrence who are most likely to benefit from investigational therapies and demonstrate treatment effects more rapidly.
Multiple technologies have emerged for MRD detection, with studies like the TOMBOLA trial in bladder cancer comparing droplet digital PCR (ddPCR) and whole-genome sequencing (WGS) approaches. This trial found an 82.9% concordance between methods across 1,282 paired plasma samples, with ddPCR showing higher sensitivity in low tumor fraction samples [16]. Emerging technologies like MUTE-Seq leverage engineered FnCas9 to selectively eliminate wild-type DNA, enabling highly sensitive detection of low-frequency cancer-associated mutations for MRD assessment [16].
Biomarker-Driven Patient Stratification
Liquid biopsy enables real-time molecular profiling for patient stratification in targeted therapy trials. The ROME trial demonstrated that combining tissue and liquid biopsy increased detection of actionable alterations despite only 49% concordance between the methods [16]. Patients receiving therapy guided by this combined approach showed improved survival outcomes, highlighting the importance of comprehensive biomarker assessment [16].
In the ACCELERATE trial for non-small cell lung cancer (NSCLC), plasma ctDNA testing provided significantly faster results than standard tissue diagnosis while identifying actionable targets missed by tissue biopsy alone [57]. This acceleration of molecular profiling can substantially reduce time-to-treatment initiation in clinical trials, particularly for cancers with aggressive biology where delays impact outcomes.
Experimental Protocols and Methodologies
ctDNA Analysis for Treatment Response Monitoring
Protocol Objective: To quantify changes in circulating tumor DNA (ctDNA) levels for assessing treatment response in solid tumor clinical trials.
Sample Collection and Processing:
- Collect 10-20 mL of peripheral blood into cell-stabilizing tubes (e.g., Streck Cell-Free DNA BCT or PAXgene Blood cDNA tubes) to prevent genomic DNA contamination and preserve sample integrity [43].
- Process samples within 2-6 hours of collection by double centrifugation (e.g., 800-1600 × g for 10 minutes, then 14,000-20,000 × g for 10 minutes) to isolate platelet-poor plasma [43].
- Store plasma at -80°C until DNA extraction to maintain cfDNA stability [43].
- Extract cell-free DNA using commercially available kits (e.g., QIAamp Circulating Nucleic Acid Kit) with elution in low-EDTA TE buffer or molecular grade water [43].
ctDNA Quantification and Analysis:
- Quantify ctDNA using tumor-informed assays where available. The Signatera assay (used in AMPLIFY-201 trial) utilizes patient-specific mutations identified through tumor tissue sequencing to create custom ctDNA detection panels [56].
- For tumor-agnostic approaches, utilize methylation-based assays or cancer-specific mutation panels targeting genes commonly mutated in the cancer type under investigation [20].
- Apply next-generation sequencing with unique molecular identifiers (UMIs) for error-suppressed sequencing, achieving sensitivity down to 0.01% variant allele frequency [58].
- Analyze sequencing data with specialized bioinformatics pipelines for variant calling, accounting for cfDNA fragmentation patterns and background sequencing noise [58].
Data Interpretation:
- Calculate variant allele frequency (VAF) for target mutations and monitor changes from baseline.
- Define molecular response as >50% reduction in mean VAF or ctDNA concentration; molecular progression as >50% increase [56].
- Correlate ctDNA dynamics with radiographic assessments and other biomarkers (e.g., serum protein biomarkers, circulating tumor cells).
Multi-Cancer Early Detection in Screening Trials
Protocol Objective: To implement multi-cancer early detection (MCED) assays in cancer screening and prevention clinical trials.
Sample Preparation for MCED Assays:
- Collect 30 mL of blood to ensure sufficient cfDNA yield, particularly for early-stage cancers with low ctDNA fraction [57].
- Use identical collection tubes and processing protocols across all clinical sites to minimize pre-analytical variability [57].
- Extract cfDNA with attention to fragment size distribution preservation, as methylation patterns influence cfDNA fragmentation [20].
Methylation Profiling and Analysis:
- Process cfDNA using whole-genome bisulfite sequencing (WGBS) or reduced representation bisulfite sequencing (RRBS) for discovery-phase methylation profiling [20].
- For targeted validation, employ bisulfite conversion followed by PCR amplification of cancer-specific methylated regions.
- Analyze methylation patterns using machine learning classifiers trained to identify cancer-specific methylation signatures and predict tissue of origin [16] [20].
- The Galleri test (GRAIL) methodology involves assessing methylation patterns at approximately 1 million CpG sites to detect cancer signals and predict cancer origin [57].
Data Interpretation and Follow-up:
- Classify samples as "cancer signal detected" or "cancer signal not detected" based on methylation classifier scores.
- For positive signals, provide cancer signal origin (CSO) prediction to guide diagnostic workup.
- In the PATHFINDER study, the CSO classifier achieved 88.2% top prediction accuracy (93.6% when considering top two predictions) [16].
- Coordinate with standard diagnostic pathways for confirmatory testing of MCED-positive results.
Table 2: Multi-Cancer Early Detection Assay Performance
| Parameter | Galleri Test (PATHFINDER) | Hybrid-Capture Methylation Assay | Proteomic Analysis (EPIC Cohort) |
|---|---|---|---|
| Overall Sensitivity | Not specified | 59.7% | 19 proteins associated with premenopausal breast cancer risk [16] |
| Late-Stage Sensitivity | Not specified | 84.2% | Not specified |
| Specificity | Not specified | 98.5% | Not specified |
| Cancer Signal Origin Accuracy | 88.2% (top prediction) [16] | Not specified | Not applicable |
| Cancers Without Screening Tests | 74% of detected cancers [57] | 73% sensitivity for cancers without standard screening [16] | Not applicable |
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Research Reagents for Liquid Biopsy Applications
| Reagent/Kit | Primary Function | Application in Clinical Trials |
|---|---|---|
| Signatera MRD Test (Natera) | Tumor-informed ctDNA detection and monitoring | Custom panels for individual patients; used in AMPLIFY-201 to detect MRD and assess treatment response [56] |
| TruSight Oncology ctDNA v2 (Illumina) | Comprehensive ctDNA sequencing panel | Detection of variants in 55-600 genes (depending on configuration) for therapy selection and resistance monitoring [58] |
| CellSearch System | Circulating Tumor Cell enumeration | FDA-cleared for CTC quantification in metastatic breast, colorectal, and prostate cancer; prognostic assessment [42] |
| QIAamp Circulating Nucleic Acid Kit | cfDNA extraction from plasma | High-quality DNA extraction with minimal contamination for downstream NGS applications [43] |
| Epigenomic Enzymatic Methylation Conversion Kits | Bisulfite-free methylation analysis | Preservation of DNA integrity while converting unmethylated cytosines for methylation sequencing [20] |
Innovative Trial Designs Enabled by Liquid Biopsy
Adaptive and Enrichment Designs
Liquid biopsy facilitates innovative adaptive trial designs that can respond to molecular findings in real-time. The ability to rapidly assess pharmacodynamic effects through ctDNA reduction allows for dose escalation decisions within weeks rather than waiting months for radiographic endpoints [56]. This approach was successfully implemented in the AMPLIFY-201 trial, where ctDNA dynamics informed dose selection for the cancer vaccine candidate ELI-002 [56].
Liquid biopsy also enables risk-based enrichment in adjuvant therapy trials. By selecting patients with positive ctDNA after surgery, sponsors can create study populations with higher recurrence risk, leading to smaller, faster trials with higher event rates [56]. The ongoing randomized Phase II trial of ELI-002 (NCT05726864) separately enrolls pancreatic cancer patients with and without positive baseline liquid biopsy tests, allowing for correlation of ctDNA clearance with disease-free survival in the high-risk group [56].
Integrated Biomarker Strategies
Advanced trial designs now incorporate multiple liquid biopsy analytes to comprehensively understand drug mechanism of action. The AMPLIFY-201 study exemplifies this approach by integrating ctDNA measurements with T-cell response assays and serum protein biomarkers [56]. This multi-analyte strategy demonstrated a significant correlation between the mechanism of action (T-cell induction) and antitumor effects (ctDNA reduction), providing compelling evidence for biological activity during early-phase development [56].
The CIRI-LCRT model presented at AACR 2025 illustrates how liquid biopsy can be combined with other data modalities to enhance predictive power. This composite score integrated radiomic features from post-chemoradiation CT scans with serial ctDNA measurements to predict progression in NSCLC patients a median of 2-3 months earlier than conventional MRD assays [16].
Analytical Considerations and Challenges
Standardization and Validation
The lack of standardized protocols remains a significant challenge in implementing liquid biopsy across multi-center clinical trials [57]. Variations in sample collection tubes, processing timelines, DNA extraction methods, and analysis platforms can introduce substantial variability that impacts result interpretation [43] [57]. To address these challenges, trial protocols should specify detailed standard operating procedures for:
- Blood collection: Consistent tube types, fill volumes, and inversion procedures
- Sample processing: Centrifugation speed, duration, and temperature controls
- Storage conditions: Plasma aliquoting, freeze-thaw cycles, and storage duration limits
- DNA extraction: Validated kits with quality control metrics including DNA yield, fragment size distribution, and absence of genomic DNA contamination
Analytical validation should establish sensitivity, specificity, precision, and limit of detection for each intended use [43]. For MRD detection, the LOD95 (limit of detection at 95% probability) should be established across the expected ctDNA fraction range, with technologies like uRARE-seq achieving LOD95 of 0.05% in urine samples for bladder cancer monitoring [16].
Interpretation in Low Tumor Fraction Contexts
Sensitivity limitations present particular challenges in early-stage disease and certain cancer types where ctDNA fractions are low [20]. Cancers of the central nervous system and early-stage disease often have ctDNA fractions below 0.1%, requiring highly sensitive technologies for reliable detection [20]. The MUTE-Seq method addresses this challenge through engineered FnCas9 to selectively eliminate wild-type DNA, thereby enriching for mutant alleles and improving detection sensitivity [16].
Bioinformatic approaches also help overcome sensitivity limitations. Methods that analyze fragmentomics - the size distribution and fragmentation patterns of cfDNA - can distinguish cancerous from non-cancerous DNA even at low tumor fractions [16]. One study demonstrated that cfDNA fragmentome analysis could identify liver cirrhosis with high accuracy (AUC=0.92) in a 724-person cohort, enabling earlier intervention in high-risk populations [16].
Liquid biopsy has evolved from a promising concept to an essential tool in oncology drug development, providing unprecedented insights into tumor dynamics and treatment response [43]. The integration of ctDNA, CTC, and methylation analyses into clinical trials enables more efficient trial designs, accelerated dose optimization, and deeper understanding of drug mechanism of action [56] [20]. As technologies continue advancing with improved sensitivity and multi-omic approaches, liquid biopsy is poised to become fully integrated across the drug development continuum - from first-in-human studies to post-marketing surveillance - ultimately accelerating the delivery of more effective therapies to cancer patients.
Overcoming Technical Challenges and Optimizing Liquid Biopsy Performance
Addressing Low ctDNA Shedding and Tumor Fraction Variability
Liquid biopsy, particularly the analysis of circulating tumor DNA (ctDNA), has emerged as a transformative tool in oncology research and drug development. However, its utility is often challenged by biological and technical factors, chiefly low ctDNA shedding and significant variability in tumor fraction (TF). ctDNA tumor fraction is the proportion of total cell-free DNA (cfDNA) in a sample that is tumor-derived [59] [60]. A low TF, often defined as below 1%, can lead to false-negative results in comprehensive genomic profiling, as the signal from genuine tumor alterations is obscured by the high background of wild-type DNA [59] [6]. This document outlines standardized protocols and analytical frameworks to address these challenges, ensuring reliable data for preclinical and clinical research.
Quantitative Landscape of ctDNA Detection
Understanding the typical ranges and detection rates of ctDNA across cancer types is crucial for experimental design and data interpretation. The following table summarizes key performance data.
Table 1: Detection Rates and Concordance of Liquid Biopsy Profiling
| Cancer Type / Context | Key Metric | Performance / Value | Notes / Conditions |
|---|---|---|---|
| Paired LBx-TBx (Multi-Cancer) [59] | Positive Percent Agreement (PPA) | 63% (All samples) | Compares Liquid Biopsy (LBx) to Tissue Biopsy (TBx) |
| Negative Predictive Value (NPV) | 66% (All samples) | Compares Liquid Biopsy (LBx) to Tissue Biopsy (TBx) | |
| PPA & NPV | 98% and 97% | When ctDNA Tumor Fraction (TF) ≥1% | |
| NSCLC (Negative LBx) [59] | Driver detection on subsequent tissue | 37% | All patients with negative LBx had ctDNA TF <1% |
| Sarcoma (LP-WGS) [61] | Detection Rate in Plasma | 9 of 13 patients (69%) | Used low-passage whole-genome sequencing to detect copy number alterations |
The TF threshold is critical for interpreting negative results. Research demonstrates that a negative liquid biopsy result with a TF ≥1% is a highly reliable "informative negative," with a 97% negative predictive value that the tissue biopsy will not find a targetable driver alteration. In contrast, a negative result with TF <1% is "indeterminate," and reflex tissue testing is strongly recommended, as it identified drivers in over a third of such lung cancer cases [59] [60].
Experimental Protocols for Enhanced ctDNA Analysis
Protocol 1: Pre-analytical Sample Collection and Handling to Maximize ctDNA Yield
Accurate quantification begins with robust pre-analytical practices to prevent sample degradation and contamination with genomic DNA.
Blood Collection:
- Volume: Draw a minimum of 2 × 10 mL of blood into dedicated blood collection tubes (BCTs) for a single-analyte ctDNA assay [6].
- Equipment: Use butterfly needles, avoid excessively thin needles, and minimize tourniquet time to prevent hemolysis and the release of wild-type DNA from blood cells [6].
- Tube Selection:
- Streck cfDNA BCT, PAXgene Blood ccfDNA Tube (Qiagen), or equivalents containing cell-stabilizing preservatives are recommended. These tubes allow for sample storage and transportation at room temperature for up to 3–7 days [6].
- Conventional EDTA tubes are an alternative but require plasma separation within 2–6 hours of draw at 4°C to avoid lysis of white blood cells [6].
Plasma Processing:
- Centrifuge tubes at 1,600–2,000 × g for 10–20 minutes at 4°C to separate plasma from cellular components.
- Carefully transfer the supernatant (plasma) to a new tube without disturbing the buffy coat.
- Perform a second, higher-speed centrifugation at 16,000 × g for 10 minutes at 4°C to remove any remaining cellular debris and platelets.
- Aliquot the purified plasma into nuclease-free tubes and store at –80°C until DNA extraction.
Protocol 2: Analytical Methods to Overcome Low Tumor Fraction
This protocol describes a multiomic approach to TF estimation and mutation detection, designed to confound low TF and clonal hematopoiesis.
DNA Extraction and Quality Control:
- Extract cfDNA from plasma using silica column-based or magnetic bead-based methods, with the latter showing higher efficiency for recovering short cfDNA fragments [62].
- Quantify cfDNA using a fluorescence-based assay (e.g., Qubit). The typical yield from 10 mL of plasma is 10–100 ng, though this varies with tumor burden [62] [6].
Library Preparation and Next-Generation Sequencing (NGS):
- Use a comprehensive genomic profiling assay (e.g., FoundationOne Liquid CDx) that targets 300+ cancer-related genes and ~30,000 genome-wide SNPs [59] [60].
- The multi-modal TF estimation algorithm integrates:
- Aneuploidy Signal: A robust copy-number model analyzing genome-wide SNP allele frequencies and coverage variation [59].
- Somatic Variant Allele Frequency (VAF): The VAF of short variants and rearrangements deemed highly likely to be somatic, identified via a whitelist of variants biased against clonal hematopoiesis (CH) and fragment size analysis [59].
- Fragmentomics: Analysis of cfDNA fragment sizes to exclude CH-derived aneuploidy signal [59] [63].
Bioinformatic Analysis:
- Process sequencing data through a dedicated bioinformatics pipeline (e.g., the ichorCNA algorithm for low-pass WGS data) to estimate TF and call somatic mutations and copy-number alterations [61].
- A sample is considered to have high TF and an "informative negative" result if the calculated TF is ≥1% [59] [60].
Protocol 3: Induction of Transient ctDNA Release
For cases of ultra-low shedding tumors, physical stimulation can be used to transiently increase ctDNA levels before blood collection [6].
- Principle: Apply localized physical stress to the tumor to induce apoptosis and/or necrosis, leading to a temporary spike in ctDNA concentration in the bloodstream.
- Procedure - Localized Irradiation:
- Administer a single, low dose of localized radiation to the primary or metastatic tumor lesion.
- Schedule the blood draw for the liquid biopsy within 6–24 hours post-irradiation to capture the peak of ctDNA release [6].
- Alternative Procedure - Focused Ultrasound:
- Techniques like "sonobiopsy" employ focused ultrasound, sometimes in combination with microbubbles, to mechanically disrupt tumor cells and facilitate ctDNA release [6].
- Safety Note: These are experimental procedures and must be conducted under appropriate ethical approvals and safety guidelines.
Workflow Visualization
The following diagram summarizes the integrated experimental strategy for addressing low ctDNA shedding and TF variability.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Reagents and Kits for ctDNA Workflows
| Research Reagent / Kit | Function / Application | Key Features / Rationale for Use |
|---|---|---|
| Streck cfDNA BCT [6] | Blood Collection & Stabilization | Prevents white blood cell lysis and release of wild-type DNA; enables room-temperature transport for up to 7 days. |
| FoundationOne Liquid CDx [59] [60] | Comprehensive Genomic Profiling | FDA-approved assay; provides multiomic ctDNA TF estimation and analyzes 324 genes; critical for interpreting negative results. |
| QIAamp Circulating Nucleic Acid Kit (or equivalent) [62] [6] | cfDNA Extraction from Plasma | High-efficiency recovery of short, fragmented cfDNA using silica-membrane technology. |
| Northstar Response Assay [10] | Methylated ctDNA Quantification | Tumor-naive assay; uses quantitative counting template (QCT) technology for precise therapy response monitoring via methylation. |
| ichorCNA Algorithm [61] | Bioinformatic TF Estimation | Designed to estimate TF from low-passage whole-genome sequencing (LP-WGS) data by detecting copy number alterations. |
| Primers/Probes for ddPCR (e.g., for KRAS, EGFR) [63] [6] | Targeted Mutation Detection | Ultra-sensitive absolute quantification of specific mutations; ideal for longitudinal monitoring of known alterations. |
Successfully navigating the challenges of low ctDNA shedding and variable tumor fraction requires a holistic and multi-faceted strategy. By implementing rigorous pre-analytical protocols, employing multiomic analytical techniques that provide accurate TF quantification, and exploring innovative stimulation methods, researchers can significantly enhance the sensitivity and reliability of liquid biopsy. Adherence to these application notes will empower more robust cancer monitoring, drug development, and clinical research, ultimately strengthening the utility of liquid biopsy as a cornerstone of precision oncology.
Sensitivity and Specificity Optimization for Early-Stage Cancer Detection
Liquid biopsy (LB) represents a minimally invasive method for cancer screening and treatment response assessment by analyzing tumor-derived products released into blood or other body fluids [64]. In the era of immune-oncology, recent evidence indicates that tumor-specific immune responses can be detected in peripheral immune cells, creating opportunities for earlier detection and intervention [64]. The optimization of sensitivity (the test's ability to correctly identify cancer) and specificity (the test's ability to correctly identify non-cancer) is paramount for developing clinically useful LB tests, especially for early-stage disease when treatment can be more effective and less toxic [65].
This document provides detailed application notes and protocols framed within a comprehensive liquid biopsy workflow for cancer monitoring research, with specific focus on optimizing sensitivity and specificity parameters for early-stage detection across multiple cancer types.
Key Performance Metrics and Optimization Framework
Quantitative Performance of Current Technologies
Recent large-scale studies demonstrate the evolving performance of liquid biopsy platforms. The following table summarizes published performance characteristics of selected LB technologies for early-stage cancer detection:
Table 1: Performance Characteristics of Selected Liquid Biopsy Technologies
| Technology/Cancer Type | Early-Stage Sensitivity | Specificity | Sample Size (n) | Key Biomarkers |
|---|---|---|---|---|
| Dxcover (FTIR Spectroscopy) [65] | 2,092 (total) | |||
| • Brain | Not specified | Not specified | Not specified | FTIR spectral signature |
| • Breast | Not specified | Not specified | Not specified | FTIR spectral signature |
| • Colorectal | Not specified | Not specified | Not specified | FTIR spectral signature |
| • Kidney | Not specified | Not specified | Not specified | FTIR spectral signature |
| • Lung | Not specified | Not specified | Not specified | FTIR spectral signature |
| • Ovarian | Not specified | Not specified | Not specified | FTIR spectral signature |
| • Pancreatic | Not specified | Not specified | Not specified | FTIR spectral signature |
| • Prostate | Not specified | Not specified | Not specified | FTIR spectral signature |
| • Pooled (Stage I) | 64% (at 99% specificity) | 99% | Not specified | FTIR spectral signature |
| • Pooled (Stage I) - Tuned | 99% (at 59% specificity) | 59% | Not specified | FTIR spectral signature |
| HCC Blood Test [66] | 82% | 87% | 401 (136 cases + 265 controls in validation) | Multi-target assay |
| Methylation-Based Multi-Cancer Test [67] | Varies by cancer type | 99% | Not specified | ctDNA methylation patterns |
Mathematical Framework for Test Optimization
A quantitative framework for evaluating multi-cancer tests reveals that the expected number of individuals exposed to unnecessary confirmation (EUC) is overwhelmingly determined by test specificity, especially when disease prevalence is low [67]. The relationship between harms and benefits can be expressed mathematically:
Expected Unnecessary Confirmations (EUC): EUC = N · [ÏA · PA(T+) · (1-LA(T+)) + ÏB · PB(T+) · (1-LB(T+)) + (1-ÏA-ÏB)(1-Sp)]
Cancers Detected (CD): CD = N · (ÏA · MSA + ÏB · MSB)
Lives Saved (LS): LS = N · (mA · MSA · RA + mB · MSB · RB)
Where: N = number tested; Ï = prevalence; P(T+) = test sensitivity; L(T+) = correct localization probability; Sp = specificity; MS = marginal sensitivity; m = probability of cancer death without screening; R = mortality reduction among detected cases [67].
This framework demonstrates that harm-benefit tradeoffs improve when tests prioritize more prevalent and/or lethal cancers for which curative treatments exist [67].
Experimental Protocols for Sensitivity and Specificity Optimization
Protocol: Fourier Transform Infrared (FTIR) Spectroscopy Liquid Biopsy
Principle: This pan-omic approach analyzes the full complement of tumor and immune-derived markers present within blood derivatives using spectral signatures rather than single analyte detection [65].
Sample Preparation:
- Collect peripheral blood using EDTA or citrate tubes
- Process within 2 hours of collection
- Centrifuge at 2,000 × g for 10 minutes to separate plasma
- Aliquot and store at -80°C until analysis
- Use minute sample volumes (as little as 10μL)
FTIR Spectroscopy:
- Use Fourier transform infrared spectrometer with DTGS detector
- Acquire spectra in transmission mode
- Spectral range: 4000-400 cmâ»Â¹
- Resolution: 4 cmâ»Â¹
- Accumulate 64 scans per sample
- Include quality control samples with each batch
Data Analysis and Machine Learning:
- Preprocess spectra: vector normalization, second derivative, and Savitzky-Golay smoothing
- Divide dataset: 70% training, 30% validation
- Implement machine learning algorithms (e.g., support vector machines, random forests)
- Optimize parameters through cross-validation
- Calculate area under ROC curve for performance assessment
Performance Optimization Notes: The Dxcover platform demonstrated that test performance can be fine-tuned to maximize either sensitivity or specificity depending on clinical requirements [65]. When tuned for higher sensitivity, the model identified 99% of Stage I cancers (with specificity 59%), while maintaining 99% specificity detected 64% of Stage I cancers [65].
Protocol: Circulating Biomarker Isolation and Analysis
Principle: Isolate and analyze various tumor-derived components including circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), tumor extracellular vesicles (EVs), and tumor-educated platelets (TEPs) [31] [64].
CTC Enrichment and Detection:
- Use antibody-based capture (e.g., EpCAM) or size-based filtration
- Implement immunocytochemistry for identification (CK8,18,19+, CD45-)
- Perform single-cell whole genome or transcriptome analysis
ctDNA Extraction and Analysis:
- Extract cell-free DNA from plasma using silica-membrane columns
- Quantify using fluorometric methods
- Analyze through:
- Targeted sequencing for mutation detection
- Methylation profiling using bisulfite treatment
- Fragmentomics analysis utilizing whole-genome sequencing
Extracellular Vesicle Isolation:
- Use preparative ultracentrifugation (≥50% of EV isolation methods)
- Alternative: nanomembrane ultrafiltration concentrators
- Characterize by nanoparticle tracking analysis, electron microscopy
- Analyze EV cargo (proteins, nucleic acids) via proteomics, next-generation sequencing
Tumor-Educated Platelets:
- Isclude platelets from blood samples using differential centrifugation
- Extract RNA for sequencing analysis
- Analyze splicing patterns and RNA profiles for cancer signals
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Research Reagents for Liquid Biopsy Workflows
| Reagent/Resource | Function | Application Notes |
|---|---|---|
| Cell-Free DNA Blood Collection Tubes | Stabilizes nucleated blood cells to prevent genomic DNA contamination | Critical for accurate ctDNA analysis; enables sample transport |
| Silica-Membrane Extraction Kits | Isolate and purify cell-free nucleic acids from plasma | High purity essential for downstream sequencing |
| EpCAM-Coated Magnetic Beads | Immunoaffinity capture of circulating tumor cells | Enables CTC enrichment from whole blood |
| Ultracentrifugation Equipment | Isolation of extracellular vesicles based on size/density | Current gold standard for EV separation; reduces contamination |
| Bisulfite Conversion Kits | DNA treatment for methylation analysis | Identifies epigenetic alterations in ctDNA |
| Multiplex PCR Panels | Amplification of cancer-associated mutations | Enables simultaneous detection of multiple variants |
| Next-Generation Sequencing Libraries | Preparation of nucleic acids for high-throughput sequencing | Comprehensive mutation and methylation profiling |
| Machine Learning Algorithms | Pattern recognition in complex datasets | Identifies subtle signatures in spectral or sequencing data |
Workflow Integration and Optimization Strategies
The following diagram illustrates the integrated workflow for liquid biopsy analysis with optimization checkpoints for sensitivity and specificity:
Diagram 1: Liquid Biopsy Workflow with Optimization Checkpoints
Optimization Strategies for Enhanced Performance
Multi-Modal Approach: Combine independent biomarker classes (ctDNA, EVs, proteins) to maximize sensitivity while maintaining specificity [65] [64]. The pan-omic approach of the Dxcover platform analyzing the full complement of markers demonstrates this principle [65].
Stage-Specific Optimization: Develop and validate algorithms specifically on early-stage cancer samples, as sensitivity is typically lower for early versus advanced-stage tumors [67].
Tunable Thresholds: Implement adjustable decision boundaries that can be optimized for either high-sensitivity (screening) or high-specificity (confirmatory) applications based on clinical context [65].
Localization Verification: For multi-cancer tests, incorporate tissue-of-origin localization as a specificity checkpoint, as incorrect localization can lead to unnecessary diagnostic procedures [67].
The following diagram illustrates the decision pathway for optimizing the balance between sensitivity and specificity based on clinical application:
Diagram 2: Clinical Context Determines Performance Optimization Strategy
Optimizing sensitivity and specificity in liquid biopsy for early cancer detection requires a multifaceted approach incorporating multi-analyte strategies, advanced computational methods, and clinical context-aware threshold setting. The protocols and frameworks presented here provide researchers with validated methodologies to advance the translational potential of liquid biopsies in cancer monitoring research. As the field evolves, standardization of these approaches across laboratories will be essential for realizing the full clinical potential of liquid biopsy technologies.
Standardization of Pre-analytical and Analytical Procedures
The integration of liquid biopsy into clinical and research workflows for cancer monitoring represents a paradigm shift in precision oncology. Unlike traditional tissue biopsies, liquid biopsy enables the minimally invasive detection and analysis of circulating tumor-derived biomarkers, such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and extracellular vesicles, from bodily fluids like blood [43]. This capability permits real-time monitoring of tumor dynamics, treatment response, and the emergence of drug resistance [68]. However, the translational potential of liquid biopsy is heavily dependent on the rigorous standardization of pre-analytical and analytical procedures [43]. Variability in these early stages can significantly impact the sensitivity, specificity, and overall reliability of downstream analyses, potentially leading to inconsistent or erroneous results. Therefore, establishing robust, standardized protocols is a critical prerequisite for generating high-quality, reproducible data that can inform drug development and clinical decision-making [69] [70].
This document outlines detailed application notes and protocols for standardizing the pre-analytical and analytical phases of a liquid biopsy workflow, specifically contextualized within cancer monitoring research.
Standardized Pre-analytical Phase
The pre-analytical phase encompasses all processes from patient preparation to the point where the sample is ready for analysis. A majority of errors in laboratory medicine originate in this phase, making its standardization paramount [70].
Key Pre-analytical Variables and Protocols
The following variables require strict control and standardization. An overview of the entire pre-analytical workflow is provided in Figure 1.
Figure 1: Pre-analytical Workflow for Liquid Biopsy Specimens.
Patient Preparation and Sample Collection: Clear instructions must be provided to and followed by patients and phlebotomists. This includes dietary restrictions (e.g., fasting for certain analytes), adherence to a specific time of day for collection (e.g., due to diurnal variations of some biomarkers), and drug restrictions where applicable [70]. For blood collection, which is the most common source, standard venepuncture procedures must be followed. Key considerations include minimizing venous stasis, using the correct tube type (e.g., specialized cell-free DNA collection tubes for ctDNA, EDTA tubes for CTCs), and drawing a sufficient volume for the intended analyses [43] [70]. The "order of draw" should be respected to minimize cross-contamination between tube additives.
Sample Handling, Transport, and Storage: After collection, the time and temperature before processing are critical. For ctDNA analysis, whole blood should be processed within a defined window (e.g., within 2-6 hours of draw when using EDTA tubes) to prevent leukocytic DNA contamination from hemolysis or cell lysis [70]. Centrifugation conditions (speed, time, temperature) must be optimized and standardized for the target biomarker—typically a double-centrifugation protocol is used to obtain platelet-poor plasma for ctDNA analysis [43]. Post-processing, plasma aliquots should be stored at -80 °C to preserve analyte integrity and minimize freeze-thaw cycles, which can degrade nucleic acids [70].
Pre-analytical Quality Control and Accept/Reject Criteria
Explicit accept/reject criteria for incoming specimens must be established to ensure only suitable samples are processed [69] [70]. These criteria should be analyte-specific but generally include:
- Assessment of Hemolysis: Visually or via spectrophotometry; severe hemolysis can interfere with assays and release genomic DNA, diluting the tumor fraction of ctDNA [70].
- Verification of Tube Type and Additive: Confirming the correct collection tube was used (e.g., rejecting serum tubes for ctDNA analysis).
- Sample Volume Sufficiency: Ensuring sufficient volume is available for the designated tests.
- Integrity of Sample Labeling: Confirming accurate patient identification and collection time/date.
- Adherence to Time/Temperature Logs: Verifying that transport and storage conditions were within specification.
Table 1: Summary of Critical Pre-analytical Variables for Blood-Based Liquid Biopsy
| Variable Category | Specific Parameter | Standardized Protocol Example | Potential Impact of Deviation |
|---|---|---|---|
| Patient Preparation | Fasting | Collect after 6-8 hour fast for nucleosome stability studies | Altered baseline levels of metabolites/nucleosomes |
| Time of Day | Morning draw (e.g., 0800) for standardized comparisons | Introduction of diurnal variation bias | |
| Sample Collection | Tube Type | 10mL Streck cfDNA BCT or K2EDTA tubes | Inadequate preservation of ctDNA; cell lysis |
| Volume | Two 10mL tubes minimum for biomarker discovery | Insufficient material for replicate/backup assays | |
| Order of Draw | Plain → Citrate → Heparin → EDTA → Fluoride [70] | Additive cross-contamination | |
| Sample Handling | Time to Process | <2hrs (EDTA); <72hrs (cfDNA BCT) at room temp | False positives from wild-type genomic DNA release |
| Centrifugation | 1,600 x g for 10 min, then 16,000 x g for 10 min (4°C) | Incomplete cell removal; poor plasma yield | |
| Sample Storage | Temperature | Long-term storage at -80°C in multiple aliquots | Analyte degradation (ctDNA fragmentation) |
| Freeze-Thaw Cycles | Maximum of 2 cycles for ctDNA | Degradation of nucleic acid targets |
Standardized Analytical Phase
The analytical phase begins when the processed specimen is logged into the laboratory and involves the actual testing and analysis of the sample [69]. Standardization here focuses on the technological platforms and procedures used to detect and characterize tumor-derived biomarkers.
Analytical Methodologies and Workflow
The choice of analytical method depends on the biomarker (ctDNA, CTCs, exosomes) and the clinical or research question (e.g., mutation detection, genome-wide profiling). A generalized analytical workflow is depicted in Figure 2.
Figure 2: Generalized Analytical Workflow for Liquid Biopsy.
Biomarker Isolation and Extraction: For ctDNA, this involves extracting cell-free DNA from plasma using silica-membrane column-based or magnetic bead-based kits optimized for low DNA concentrations and small fragment sizes [43]. For CTCs, enrichment techniques include label-dependent methods (e.g., immunoaffinity capture using EpCAM antibodies) or label-independent methods (e.g., size-based filtration, density gradient centrifugation) [43]. Exosomes are typically isolated via ultracentrifugation, precipitation, or immunoaffinity capture. Each method requires standardized protocols to ensure consistent yield and purity.
Analytical Detection Technologies: The core of liquid biopsy analysis.
- Next-Generation Sequencing (NGS): This is the cornerstone for comprehensive ctDNA profiling, allowing for the simultaneous detection of multiple mutations, copy number alterations, and fusions across many genes [43]. Standardization involves using uniform library preparation kits, target enrichment panels (e.g., hybrid capture or amplicon-based), and sequencing platforms with controlled coverage depth and quality metrics.
- Digital PCR (dPCR): Including droplet digital PCR (ddPCR) and BEAMing, these methods offer ultra-sensitive detection and absolute quantification of known, specific mutations (e.g., for monitoring minimal residual disease) [43]. They are highly precise but low-plex. Standardization involves consistent assay design, partition generation, and threshold setting.
Analytical Quality Control and Assurance
Robust quality control (QC) is embedded throughout the analytical phase to monitor performance and ensure result validity [69].
- Implementation of Standard Operating Procedures (SOPs): Detailed, written SOPs must govern every process, from nucleic acid quantification to instrument operation [69].
- Use of Controls: Each run should include:
- Positive Controls: Synthetic or cell line-derived materials with known mutations to confirm assay sensitivity.
- Negative Controls: Confirmed wild-type samples to monitor for contamination.
- Internal Controls: To assess the efficiency of extraction and amplification.
- Validation and Calibration: All instruments (sequencers, PCR machines, centrifuges) must undergo regular calibration and preventive maintenance. Analytical methods require thorough validation before implementation, establishing performance characteristics like analytical sensitivity (limit of detection), specificity, precision, and accuracy [69].
- Personnel Training and Competency Evaluation: Staff must be rigorously trained on all SOPs and their competency periodically assessed [69].
Table 2: Key Analytical Techniques and Quality Metrics for Liquid Biopsy
| Analytical Target | Primary Technique(s) | Key Performance Metrics | QC Measures |
|---|---|---|---|
| ctDNA (Targeted Mutations) | Digital PCR (dPCR/ddPCR) | Limit of Detection (LOD) (<0.1%), Absolute Quantification | No Template Control (NTC), Positive Control, Inter-/Intra-assay Precision |
| ctDNA (Broad Panel) | Next-Generation Sequencing (NGS) | Sensitivity/Specificity, Mean Coverage Depth (>5000x), Duplication Rate | PhiX Control, Unique Molecular Indexes (UMIs), Sample/Index Cross-contamination Check |
| Circulating Tumor Cells (CTCs) | Immuno-magnetic Enrichment + Microscopy/IF-FISH | Cell Recovery Rate, Purity (WBC count), Enumeration | Spike-in Control Cells (e.g., SkBr3, PC3), Antibody Staining Specificity |
| Extracellular Vesicles/Exosomes | Ultracentrifugation/Precipitation + RNA-seq | Particle Concentration (NTA), RNA Integrity Number (RIN) | Western Blot for CD63/TSG101, Negative Staining Markers |
The Scientist's Toolkit: Essential Research Reagent Solutions
Successful execution of standardized liquid biopsy workflows relies on a suite of essential reagents and materials. The following table details key solutions for a protocol focused on ctDNA analysis via NGS.
Table 3: Essential Research Reagent Solutions for ctDNA NGS Workflow
| Item | Function/Application | Critical Specifications |
|---|---|---|
| Cell-Free DNA Blood Collection Tubes | Stabilizes nucleated blood cells for up to 14 days, preventing lysis and release of genomic DNA that dilutes ctDNA [43]. | Plasma cfDNA yield and stability over time; compatibility with downstream extraction kits. |
| Magnetic Bead-based cfDNA Extraction Kits | Isolation of high-quality, short-fragment cfDNA from plasma; removes PCR inhibitors and proteins. | DNA yield & purity (A260/A280); efficiency for <200bp fragments; elution volume. |
| Library Preparation Kit for Low-Input DNA | Preparation of sequencing libraries from low-concentration, fragmented cfDNA inputs (e.g., 5-50ng). | Input DNA requirement; compatibility with Unique Molecular Indexes (UMIs); conversion efficiency. |
| Hybrid-Capture Target Enrichment Panels | Biotinylated probes designed to capture and enrich specific genomic regions (e.g., cancer gene panels) from the whole library. | Panel size and content; on-target rate; uniformity of coverage; ability to detect fusions/CNVs. |
| UMI Adapters | Short, unique nucleotide sequences added to each original DNA molecule during library prep to tag and correct for PCR and sequencing errors. | Complexity (diversity); error rate reduction; accurate quantification of original molecules. |
| Validated Positive Control Reference Material | Synthetic or fragmented genomic DNA with known mutations at defined variant allele frequencies (VAFs) for assay validation and run QC. | Certified VAF (e.g., 1%, 0.1%, 5%); commutability with patient samples; material format (e.g., synthetic, cell-line derived). |
Liquid biopsy, the analysis of tumor-derived material in blood, has emerged as a powerful, minimally invasive tool for cancer genomics, therapy selection, and disease monitoring [71] [42]. However, its clinical utility is compromised by several sources of false positive results, which can lead to incorrect genotype assignment and misguide treatment decisions. Among these, clonal hematopoiesis of indeterminate potential (CHIP) represents a major confounding factor [72] [73]. CHIP is an age-related expansion of blood cell progenitors carrying somatic mutations, without an apparent hematological malignancy [72]. As most cell-free DNA (cfDNA) originates from hematopoietic cells, CHIP-derived mutations can be detected in plasma cfDNA sequencing and mistakenly interpreted as tumor-derived somatic variants (circulating tumor DNA, or ctDNA) [73] [74]. This application note details protocols and strategies to identify, manage, and bioinformatically filter CHIP and other common sources of false positives to ensure the analytical validity of liquid biopsy results in cancer research.
Quantitative Landscape of Interfering Mutations
Understanding the prevalence and typical genetic landscape of CHIP is the first step in managing its impact. The following tables summarize key quantitative data from recent studies.
Table 1: CHIP Prevalence in Metastatic Cancer Patients [73]
| Cancer Type | Sample Size (N) | CHIP Prevalence (VAF ≥0.25%) | CHIP Prevalence (VAF ≥2%) | CHIP Prevalence (VAF ≥10%) |
|---|---|---|---|---|
| Metastatic Urothelial Carcinoma (mUC) | 115 | 76% | ~34% (combined) | ~13% (combined) |
| Metastatic Renal Cell Carcinoma (mRCC) | 184 | 71% | ~34% (combined) | ~13% (combined) |
| Combined Cohort | 299 | 73% | 34% | 13% |
Table 2: Genes Frequently Mutated in CHIP and Their Potential for Interference [72] [73] [74]
| Gene Category | Example Genes | Potential for ctDNA Interference | Notes |
|---|---|---|---|
| Epigenetic Regulators | DNMT3A, TET2, ASXL1 (DTA genes) |
Lower | Less frequent drivers in solid tumors; often excluded from ctDNA panels. |
| DNA Damage Response | ATM, CHEK2, TP53, BRCA1/2, PPM1D |
High | Core oncology genes; critical for PARPi and other therapy decisions. |
| Signaling Kinases | KRAS, IDH1, IDH2 |
High | Actionable drivers in many solid tumors. |
Table 3: Differentiating Features of CHIP vs. ctDNA Mutations
| Feature | CHIP-derived Mutation | True Tumor-derived (ctDNA) Mutation |
|---|---|---|
| Primary Source | White Blood Cells (Hematopoietic) | Tumor Cells (from primary or metastatic sites) |
| Correlation in Matched Assays | Present in WBC DNA and plasma cfDNA | Present in plasma cfDNA, absent in WBC DNA |
| Typical VAF in cfDNA | Often low (<2%), but can be high [74] | Variable, correlates with tumor fraction |
| Impact of Therapy | PPM1D CHIP expands after platinum chemotherapy [73] |
Somatic mutations change under selective pressure of cancer therapy |
| Clinical Consequence | False positive genotyping for solid tumor | True representation of tumor genome |
Experimental Protocols for CHIP Identification and Filtering
Protocol: Matched White Blood Cell (WBC) Sequencing for Comprehensive CHIP Filtering
This is the gold-standard method for identifying and removing CHIP-associated variants from plasma cfDNA results.
Principle: Sequencing matched WBC DNA from the same blood draw as the plasma sample allows for the direct identification of somatic mutations present in the hematopoietic system. Any variant detected in both the WBC DNA and the plasma cfDNA is considered a CHIP-derived "false positive" for solid tumor genotyping [73].
Materials:
- Whole blood collection tubes (e.g., EDTA or Streck tubes)
- Centrifuge with temperature control
- DNA extraction kits for plasma and buffy coat
- Targeted NGS panel covering genes of interest
- High-throughput sequencer
Procedure:
- Blood Collection and Processing:
Nucleic Acid Extraction and Sequencing:
- Extract cfDNA from plasma using a commercial kit.
- Extract high-molecular-weight genomic DNA from the buffy coat.
- Prepare sequencing libraries for both cfDNA and WBC DNA using an identical targeted NGS panel.
- Sequence the WBC DNA to a deduplicated depth of at least 25% of the cfDNA sequencing depth. For example, if cfDNA is sequenced to 4000x coverage, sequence WBC DNA to at least 1000x coverage [73].
Bioinformatic Analysis:
- Call variants in both the cfDNA and WBC DNA datasets using the same pipeline and variant allele frequency (VAF) threshold.
- Filtering Step: Subtract any variant found in the WBC DNA (VAF ≥ 0.25%) from the plasma cfDNA variant call list [73].
- The remaining, tumor-specific variants can be reported with high confidence.
Protocol: Bioinformatic Prediction of CHIP in Plasma-Only Sequencing
When a matched WBC sample is unavailable, bioinformatic models can provide a probabilistic assessment of CHIP origin.
Principle: Machine learning models trained on large datasets of known CHIP and true somatic variants can identify distinguishing features. These may include the specific gene mutated, sequence context, VAF, and fragmentomic patterns [74].
Procedure:
- Data Generation: Perform deep sequencing of plasma cfDNA.
- Variant Calling: Identify all somatic variants above the limit of detection.
- Model Application:
- Input variant features (e.g., gene name, VAF, motif) into a pre-trained classifier.
- The model outputs a probability score for the variant being CHIP-derived.
- Reporting: Flag variants with high CHIP probability for cautious interpretation. Annotate that these calls have a higher risk of being false positives without orthogonal confirmation.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 4: Essential Reagents and Kits for CHIP Management
| Reagent / Solution | Function | Key Considerations |
|---|---|---|
| Cell-Free DNA Blood Collection Tubes (e.g., Streck Cell-Free DNA BCT) | Stabilizes nucleated blood cells to prevent lysis and release of genomic DNA during transport and storage, preserving the original cfDNA profile. | Critical for preventing false positives from in vitro WBC lysis, which can mimic high tumor fraction. |
| cfDNA Extraction Kits (e.g., from QIAGEN, Roche, Thermo Fisher) | Isolation of high-quality, short-fragment cfDNA from plasma. | Optimized for low-input DNA and recovery of short fragments (~167 bp) to maximize ctDNA yield. |
| Targeted NGS Panels (e.g., Tempus xF, Guardant360, FoundationOne Liquid) | Multiplexed PCR or hybrid-capture-based sequencing of cancer-associated genes. | Panel design should cover genes of interest for the cancer type. Beware of panels that include common CHIP genes without a filtering strategy. |
| White Blood Cell DNA Extraction Kits | Isolation of genomic DNA from the buffy coat for matched sequencing. | Yield and quality of gDNA must be sufficient for the required sequencing depth. |
| Error-Corrected NGS Reagents | Unique Molecular Identifiers (UMIs) and library prep kits that reduce sequencing errors. | Lowers background noise, improving the detection of low-VAF true positives and reducing false positives from technical artifacts. |
The integration of liquid biopsy into cancer research and development demands rigorous quality control. CHIP is a pervasive biological phenomenon that, if unaddressed, can significantly compromise the integrity of ctDNA-based genomic data. The implementation of matched WBC sequencing provides the most robust solution for filtering CHIP, while advanced bioinformatic models offer a viable, though less definitive, alternative for plasma-only workflows. By adopting the protocols and mitigation strategies outlined in this document, researchers and drug developers can enhance the accuracy of their liquid biopsy analyses, leading to more reliable biomarker discovery, better patient stratification in clinical trials, and ultimately, more effective cancer therapies.
Liquid biopsy has emerged as a transformative tool in oncology, providing a minimally invasive window into tumor dynamics. While individual biomarkers like circulating tumor DNA (ctDNA) or circulating tumor cells (CTCs) offer valuable insights, their combination creates a powerful multimodal approach that captures complementary aspects of tumor biology [75]. This integrated strategy significantly enhances the sensitivity and comprehensiveness of cancer monitoring, profiling, and therapeutic response assessment [31] [76]. By concurrently analyzing ctDNA mutations, CTC molecular profiles, and epigenetic alterations such as DNA methylation, researchers can overcome the limitations of single-analyte approaches, including tumor heterogeneity and low analyte abundance in early-stage disease [75] [77]. This protocol details standardized methods for integrating these three analytical dimensions within a unified liquid biopsy workflow, specifically designed for cancer monitoring research.
Comparative Analysis of Liquid Biopsy Biomarkers
The three core biomarkers—ctDNA, CTCs, and epigenetic markers—provide distinct yet complementary information. ctDNA reflects tumor-specific genetic alterations, CTCs offer intact cells for functional analysis, and epigenetic markers reveal regulatory changes often detectable early in carcinogenesis [75] [42]. Their technical characteristics and clinical utilities are summarized in Table 1.
Table 1: Comparative Analysis of Core Liquid Biopsy Biomarkers
| Biomarker | Primary Analytes | Key Strengths | Primary Limitations | Research Applications |
|---|---|---|---|---|
| ctDNA | Tumor-derived DNA fragments carrying genetic mutations [42] | Short half-life (15 min-2.5h) enables real-time monitoring; captures tumor heterogeneity [7] [42] | Low concentration in early-stage disease; can be confounded by clonal hematopoiesis [6] [77] | Treatment response monitoring, minimal residual disease (MRD) detection, tracking resistance mutations [7] [78] |
| CTCs | Intact tumor cells circulating in bloodstream [75] | Enables whole-cell analysis (DNA, RNA, protein); functional studies and ex vivo culture possible [75] [76] | Extreme rarity (~1 CTC per billion blood cells); technical challenges in isolation and preservation [75] [42] | Metastasis research, drug sensitivity testing, prognostic stratification [7] [76] |
| Epigenetic Markers | DNA methylation patterns, chromatin modifications [77] | High stability; tissue-of-origin identification; often detectable before mutations [77] | Complex analysis requiring specialized bioinformatics; cell-type specific interpretation needed | Early cancer detection, cancer subtyping, monitoring epigenetic therapy responses [31] [77] |
Multimodal Integration Workflow
The synergistic integration of ctDNA, CTCs, and epigenetic markers follows a standardized workflow from sample collection through data integration. This coordinated approach maximizes informational yield from a single blood draw.
Detailed Experimental Protocols
Pre-analytical Sample Processing
Standardized sample collection and processing are critical for multimodal analysis, as pre-analytical variables significantly impact all downstream applications [6].
Blood Collection: Collect 20-30 mL peripheral blood into cell-stabilizing blood collection tubes (e.g., Streck cfDNA BCT) to preserve sample integrity [6]. Maintain consistent draw times (circadian variations affect analyte levels) [6]. Process within 6 hours of collection for optimal results.
Plasma Separation: Perform double centrifugation: first at 800×g for 10 minutes at 4°C to separate plasma from cells, followed by second centrifugation at 16,000×g for 10 minutes to remove residual platelets and debris [6]. Aliquot plasma into nuclease-free tubes and store at -80°C.
Buffy Coat Collection: Retain buffy coat layer after first centrifugation for white blood cell DNA sequencing, which is essential for distinguishing tumor mutations from clonal hematopoiesis of indeterminate potential (CHIP) [77].
ctDNA Analysis Protocol
ctDNA Extraction and Quantification
- Extract ctDNA from 4-10 mL plasma using validated commercial kits (e.g., QIAamp Circulating Nucleic Acid Kit) with carrier RNA to improve recovery of low-concentration fragments [6]. Elute in 20-50 μL nuclease-free water.
- Quantify using fluorometry (e.g., Qubit dsDNA HS Assay). Expected yields vary by cancer stage: 1-100 copies/mL in early-stage disease versus up to 1,000+ copies/mL in advanced disease [6].
Genetic and Epigenetic Analysis
Table 2: ctDNA Analysis Methods and Applications
| Method | Key Features | Detection Sensitivity | Primary Applications |
|---|---|---|---|
| Tumor-Informed ddPCR | Patient-specific mutation tracking; requires prior tumor sequencing [77] | ~0.01% variant allele frequency (VAF) [79] | MRD monitoring, recurrence detection [78] |
| Tumor-Agnostic NGS Panels | Comprehensive profiling without prior tumor knowledge [77] | 0.02%-0.1% VAF depending on sequencing depth [77] | Mutation discovery, heterogeneity assessment [7] |
| Whole-Genome Bisulfite Sequencing | Genome-wide methylation profiling; requires specialized bioinformatics [77] | Varies with coverage; ~1% for differentially methylated regions | Cancer subtyping, tissue-of-origin identification [77] |
For tumor-informed approaches, first sequence tumor tissue (if available) to identify patient-specific mutations, then design custom ddPCR assays for these targets [77]. For tumor-agnostic approaches, use hybrid-capture NGS panels (e.g., Guardant360, FoundationOne Liquid CDx) covering relevant cancer genes [7]. For methylation analysis, treat DNA with bisulfite before sequencing to convert unmethylated cytosines to uracils while preserving methylated cytosines [77].
CTC Isolation and Characterization Protocol
CTC Enrichment Strategies
CTC isolation employs either label-dependent (antibody-based) or label-independent (biophysics-based) techniques, each with distinct advantages:
EpCAM-Based Immunocapture: Use CellSearch system (FDA-approved) with anti-EpCAM magnetic beads for standardized CTC enumeration [7] [75]. This method reliably captures epithelial CTCs but may miss those undergoing epithelial-to-mesenchymal transition.
Size-Based Isolation: Employ Parsortix PC1 system or membrane filters that capture CTCs based on their larger size and rigidity compared to blood cells [7] [75]. This approach is marker-independent and preserves cell viability for downstream culture.
Negative Depletion: Remove hematopoietic cells using anti-CD45 magnetic beads, enriching untouched CTCs regardless of epithelial marker expression [75].
CTC Molecular Characterization
Immunofluorescence Staining: Identify CTCs using epithelial markers (cytokeratins CK8,18,19), absence of leukocyte marker (CD45), and nuclear staining (DAPI) [75]. Include additional markers for subtyping (e.g., HER2, ER, AR).
Molecular Analysis: For genomic profiling, perform whole-genome amplification followed by NGS on single CTCs or pools. For transcriptomics, use single-cell RNA sequencing to characterize CTC heterogeneity [75] [76].
Functional Studies: Culture isolated CTCs ex vivo using low-attachment plates and specialized media to establish CTC-derived organoids for drug sensitivity testing [75].
Data Integration and Bioinformatics Analysis
Integrate data from all three modalities using customized bioinformatics pipelines:
ctDNA Variant Calling: Use unique molecular identifiers to distinguish true low-frequency variants from sequencing artifacts. Filter against dbSNP and buffy coat sequences to exclude germline polymorphisms and CHIP mutations [77].
Methylation Analysis: Process bisulfite sequencing data with specialized tools (e.g., Bismark) for alignment and methylation extraction. Compare against reference methylation atlas for tissue-of-origin identification [77].
CTC Multi-omics Integration: Correlate genomic alterations from single CTC sequencing with protein expression and transcriptional profiles.
Cross-platform Data Fusion: Develop integrated molecular signatures that combine ctDNA mutation status, CTC phenotypic characterization, and epigenetic profiles for comprehensive disease monitoring.
Essential Research Reagents and Platforms
Table 3: Essential Research Toolkit for Multimodal Liquid Biopsy
| Category | Specific Product/Platform | Primary Function | Key Considerations |
|---|---|---|---|
| Blood Collection Tubes | Streck cfDNA BCT, PAXgene Blood ccfDNA tubes [6] | Preserve blood cell integrity and prevent background DNA release | Enable sample stability for up to 7 days at room temperature |
| Nucleic Acid Extraction | QIAamp Circulating Nucleic Acid Kit, Maxwell RSC ccfDNA Plasma Kit | Isolate high-quality ctDNA from plasma | Include carrier RNA for improved low-concentration yield |
| CTC Isolation Systems | CellSearch (EpCAM-based), Parsortix PC1 (size-based) [7] | Enumerate and isolate circulating tumor cells | Choice depends on need for marker-independent capture |
| Mutation Detection | ddPCR platforms, NGS panels (Guardant360, FoundationOne) [7] [79] | Identify and quantify tumor-specific mutations | ddPCR offers highest sensitivity for known mutations |
| Methylation Analysis | Whole-genome bisulfite sequencing kits, targeted methylation panels [77] | Profile DNA methylation patterns | Requires specialized bisulfite conversion and bioinformatics |
| Data Analysis | Custom bioinformatics pipelines incorporating UMI error correction | Integrate multi-analyte data and call low-frequency variants | Essential for distinguishing true variants from artifacts |
Application in Cancer Monitoring
This multimodal approach enables comprehensive cancer monitoring across the disease continuum:
Early Detection and Screening: Combine ctDNA mutation analysis with cancer-specific methylation signatures to enhance sensitivity for early-stage tumors where analyte concentrations are minimal [77].
Minimal Residual Disease Monitoring: Use tumor-informed ctDNA assays with patient-specific mutations for ultra-sensitive MRD detection post-treatment, complemented by CTC analysis to identify persistent disseminated disease [78] [77].
Therapeutic Response Assessment: Serial monitoring of ctDNA variant allele frequencies provides quantitative response assessment, while CTC enumeration and characterization offers insights into evolving tumor biology under therapeutic pressure [7] [76].
Resistance Mechanism Identification: Detect emerging resistance mutations in ctDNA, while parallel CTC analysis reveals phenotypic adaptations and lineage plasticity contributing to treatment failure [7] [77].
This multimodal liquid biopsy framework provides researchers with a comprehensive toolkit for advancing cancer monitoring research through integrated molecular profiling.
The Role of Artificial Intelligence and Bioinformatics in Data Analysis
Liquid biopsy has emerged as a transformative, minimally invasive approach for cancer monitoring, treatment selection, and recurrence detection. This paradigm shift from traditional tissue biopsy enables real-time tracking of tumor dynamics through the analysis of circulating biomarkers in blood and other bodily fluids [42]. The resulting data, encompassing genomic, transcriptomic, and fragmentomic profiles, presents both unprecedented opportunities and significant analytical challenges due to its volume, complexity, and low signal-to-noise ratio in early-stage disease [80] [36].
Artificial intelligence (AI) and bioinformatics now serve as indispensable tools for extracting clinically actionable insights from liquid biopsy data. Machine learning (ML) and deep learning (DL) algorithms can identify subtle patterns across multi-omics datasets that elude conventional statistical methods [81]. These computational approaches are particularly valuable for integrating liquid biopsy data with other modalities such as radiomics, digital pathology, and clinical records, creating a comprehensive view of tumor heterogeneity and evolution [82]. The synergy between AI and liquid biopsy is advancing precision oncology toward more dynamic, patient-specific cancer management strategies [80].
Key Analytical Biomarkers in Liquid Biopsy
Liquid biopsy encompasses several tumor-derived components that serve as analytical targets. Each biomarker provides complementary information about tumor biology and clinical status, with varying strengths for different applications in cancer monitoring [42].
Table 1: Key Liquid Biopsy Biomarkers and Their Clinical Applications
| Biomarker | Biological Origin | Analytical Targets | Clinical Utility in Cancer Monitoring |
|---|---|---|---|
| Circulating Tumor DNA (ctDNA) | Apoptosis/necrosis of tumor cells [42] | Somatic mutations, copy number alterations, methylation patterns [80] | Treatment response monitoring, minimal residual disease detection, tracking resistance mutations [80] [42] |
| Cell-Free DNA (cfDNA) | Primarily leukocytes and stromal cells [42] | Fragmentomics patterns, nucleosome positioning [36] | Tumor burden estimation, differentiation of benign from malignant lesions [36] |
| Circulating Tumor Cells (CTCs) | Tumor cells shed into circulation [42] | Protein markers, transcriptomes, genomic alterations [42] | Prognostic stratification, metastasis research, treatment selection [42] |
| Exosomes/Extracellular Vesicles | Active secretion by cells [83] | Proteins, lipids, nucleic acids (miRNA, cfRNA) [83] | Early detection, monitoring tumor microenvironment interactions [83] |
| cfRNA/ctRNA | Cellular transcription and release | miRNA, mRNA, non-coding RNAs [36] | Gene expression profiling, treatment response biomarkers [36] |
The pre-analytical phase is critical for reliable liquid biopsy results. Standardized protocols for blood collection, processing, and storage minimize genomic DNA contamination from leukocyte lysis, which can significantly impact assay sensitivity [36]. Blood preservation tubes employing different chemistries (osmotic stabilization, biological apoptosis prevention, or chemical crosslinking) enable sample stability for varying durations when immediate processing isn't feasible [36].
AI and Machine Learning Methodologies
Foundations of AI in Bioinformatics
AI in cancer bioinformatics encompasses multiple computational paradigms, each with distinct strengths for liquid biopsy data analysis. Supervised learning algorithms, including Support Vector Machines (SVMs) and Random Forests, are trained on labeled input-output pairs to make predictions on new data, making them well-suited for classification tasks such as cancer type discrimination or mutation calling [81] [84]. Unsupervised learning methods identify inherent patterns and structures in unlabeled data, enabling discovery of novel biomarker signatures or patient subgroups without predefined categories [84]. Reinforcement learning algorithms learn decision-making through reward/penalty feedback, showing promise for optimizing dynamic treatment strategies based on serial liquid biopsy results [84].
Deep learning architectures have demonstrated particular utility for complex liquid biopsy data. Convolutional Neural Networks (CNNs) excel at identifying spatial hierarchies in data, making them valuable for analyzing fragmentation patterns in cfDNA or spectral data from exosomes [80]. Recurrent Neural Networks (RNNs) and their variants (LSTMs, GRUs) can model temporal dependencies in longitudinal liquid biopsy samples to track disease progression or treatment response [80]. Generative Adversarial Networks (GANs) can synthesize additional training data to address class imbalance issues common in rare cancer subtypes or early-stage disease [80].
Multimodal AI Integration
Multimodal Artificial Intelligence (MMAI) represents a paradigm shift in liquid biopsy analysis by integrating heterogeneous datasets into cohesive analytical frameworks [82]. By simultaneously processing molecular data from liquid biopsy with imaging data (CT, PET, MRI), histopathology slides, and clinical records, MMAI models capture multiscale tumor heterogeneity and provide more comprehensive insights than unimodal approaches [82].
The TRIDENT initiative exemplifies this approach, integrating radiomics, digital pathology, and genomics data from metastatic non-small cell lung cancer patients to identify biomarkers of treatment response [82]. Similarly, Pathomic Fusion combines histology and genomics for improved risk stratification in glioma and renal cell carcinoma [82]. These integrated models contextualize molecular findings within anatomical and clinical frameworks, yielding more biologically plausible and clinically actionable inferences [82].
Diagram 1: MMAI integrates diverse data types for comprehensive cancer analysis.
Experimental Protocols and Applications
Protocol: AI-Enhanced ctDNA Analysis for Treatment Monitoring
Objective: To detect and quantify ctDNA mutations in plasma samples for monitoring treatment response and emerging resistance mechanisms in metastatic non-small cell lung cancer (NSCLC) [80] [42].
Materials and Reagents:
- Cell-free DNA BCT Streck tubes (10mL) or PAXgene Blood ccfDNA Tubes for blood collection [36]
- NucleoSnap and NucleoSpin kits for parallel cfDNA/cfRNA isolation [36]
- Qubit dsDNA HS Assay Kit for DNA quantification
- Bioanalyzer High Sensitivity DNA Kit for fragment size distribution analysis
- Targeted next-generation sequencing panel covering relevant driver mutations (EGFR, KRAS, ALK, etc.)
- ddPCR assays for specific mutation validation
Methodology:
- Sample Collection and Processing:
- Collect 10mL peripheral blood into preservation tubes. Process within 1 hour for EDTA tubes or within 3-7 days for Streck/PAXgene tubes stored at room temperature [36].
- Centrifuge at 1600-1900×g for 15-20 minutes at room temperature to separate plasma [36].
- Aliquot plasma and store at -80°C until nucleic acid extraction.
Nucleic Acid Extraction:
- Extract cfDNA using silica-membrane technology according to manufacturer protocols [36].
- Quantify yield using fluorometric methods (Qubit). Assess fragment size distribution via microcapillary electrophoresis (Bioanalyzer).
- Determine cfDNA purity by calculating ratio of mononucleosomal DNA (160bp) to total DNA [36].
Library Preparation and Sequencing:
- Convert 20-50ng cfDNA to sequencing libraries using commercial kits optimized for low-input degraded DNA.
- Hybridize with targeted capture panels covering cancer-associated genes.
- Sequence on Illumina platform to achieve minimum 10,000X raw coverage.
Bioinformatic Analysis:
- Align sequencing reads to reference genome (BWA-MEM, Bowtie2).
- Perform duplicate marking, base quality recalibration, and local realignment (GATK).
- Call somatic variants using specialized ctDNA callers (MuTect, VarDict).
- Apply unique molecular identifiers (UMIs) to correct for PCR and sequencing errors.
AI-Enhanced Variant Interpretation:
- Implement ensemble machine learning model combining:
- Random Forest classifier trained on fragmentomics features (size distribution, end motifs)
- Deep learning model (CNN) analyzing sequence context around putative variants
- Anomaly detection algorithm identifying emerging resistance mutations
- Apply threshold optimization based on training data with known variant allele frequencies
- Implement ensemble machine learning model combining:
Longitudinal Monitoring:
- Track variant allele frequencies across multiple timepoints
- Apply change-point detection algorithms to identify significant shifts in ctDNA levels
- Correlate molecular response with radiographic findings and clinical assessments
Validation:
- Confirm low-frequency variants (<1% VAF) using orthogonal ddPCR assays
- Establish limit of detection and quantification using serially diluted reference standards
- Compare with tissue biopsy results when available
Protocol: Quantum Machine Learning for Exosome Characterization
Objective: To distinguish cancer-derived exosomes from healthy controls using quantum-inspired machine learning algorithms analyzing electrokinetic properties [83].
Materials and Reagents:
- Norgen cf-DNA/cf-RNA Preservative Tubes for plasma preservation
- ExoQuick or similar polymer-based exosome precipitation reagent
- Nanoparticle tracking analysis system (NanoSight)
- Tunable resistive pulse sensing (TRPS) instrumentation
- Quantum processing unit or quantum simulator software (Qiskit, Pennylane)
Methodology:
- Exosome Isolation and Characterization:
- Isolate exosomes from 1-2mL plasma using precipitation or size-exclusion chromatography
- Characterize particle concentration and size distribution via nanoparticle tracking
- Confirm exosomal markers (CD63, CD81) by western blot or flow cytometry
Electrokinetic Profiling:
- Measure zeta potential and surface charge properties of exosomes
- Analyze particle mobility under electrical fields using microfluidic systems
- Collect time-series data on particle movement and distribution
Quantum Machine Learning Implementation:
- Encode electrokinetic features into quantum states using amplitude encoding
- Apply quantum variational classifier with parameterized quantum circuits
- Optimize using quantum approximate optimization algorithm (QAOA)
- Compare performance with classical SVM and Random Forest models
Model Validation:
- Perform k-fold cross-validation with independent sample sets
- Assess generalizability across cancer types (colorectal, lung, bladder)
- Calculate performance metrics (AUC, sensitivity, specificity)
Table 2: Performance Comparison of AI Models in Liquid Biopsy Applications
| Application | AI Model | Performance Metrics | Clinical Context | Reference |
|---|---|---|---|---|
| Exosome Classification | Quantum Machine Learning | Superior to classical methods in distinguishing cancer vs healthy exosomes [83] | Early detection of colorectal cancer | [83] |
| Lung Cancer Risk Prediction | Sybil AI | AUC 0.92 for lung cancer risk prediction from LDCT [82] | Screening in high-risk populations | [82] |
| Melanoma Relapse Prediction | MUSK Transformer | AUC 0.833 for 5-year relapse prediction [82] | Immunotherapy response monitoring | [82] |
| Drug Sensitivity Prediction | Multimodal DREAM Challenge | Outperformed unimodal approaches [82] | Breast cancer cell lines | [82] |
| Radiomics-Liquid Biopsy Integration | CNN + Random Forest | Improved early-stage detection sensitivity [80] | Lung cancer screening | [80] |
Successful implementation of AI-enhanced liquid biopsy analysis requires both wet-lab reagents and computational resources. The following toolkit outlines essential components for establishing a robust workflow.
Table 3: Research Reagent Solutions for AI-Enhanced Liquid Biopsy
| Category | Product/Resource | Specifications | Application Notes |
|---|---|---|---|
| Blood Collection Tubes | Cell-Free DNA BCT Streck Tubes | 10mL, 14-day stability at RT, chemical crosslinking chemistry [36] | Minimizes leukocyte lysis; enables sample transport |
| Blood Collection Tubes | PAXgene Blood ccfDNA Tubes | 10mL, 14-day RT stability, biological apoptosis prevention [36] | Alternative preservation chemistry |
| Blood Collection Tubes | Norgen cf-DNA/cf-RNA Preservative Tubes | 8.4mL, 30-day RT stability, osmotic cell stabilization [36] | Highest plasma volume yield; DNA/RNA preservation |
| Nucleic Acid Extraction | NucleoSnap/NucleoSpin kits | Parallel cfDNA/cfRNA isolation from single sample [36] | Maximizes biomarker yield from limited samples |
| Quality Control | Bioanalyzer High Sensitivity DNA Kit | Fragment size analysis (50-7000bp range) [36] | Essential for cfDNA purity assessment |
| Quality Control | Qubit dsDNA HS Assay | Fluorometric quantification for low-concentration samples [36] | More accurate than spectrophotometry for cfDNA |
| Sequencing | Targeted Hybrid Capture Panels | Customizable cancer gene content; UMI incorporation | Optimized for ctDNA mutation detection |
| Computational Framework | Python Data Science Stack | NumPy, Pandas, Scikit-learn, TensorFlow/PyTorch [85] | Standard ML/DL implementation |
| Computational Framework | MONAI Medical Imaging AI | PyTorch-based framework for medical AI [82] | Specialized for radiology/pathology integration |
| Quantum ML | Qiskit/Pennylane | Quantum algorithm development platforms [83] | For quantum-inspired ML approaches |
Data Analysis and Visualization Framework
Quantitative Data Analysis Workflow
Analysis of liquid biopsy data requires a systematic approach to transform raw numerical data into clinically interpretable results. The process encompasses multiple phases, each with specific analytical considerations [84] [85].
Phase 1: Problem Definition and Research Design
- Define clear analytical objectives with measurable outcomes
- Establish appropriate study populations and sampling strategies
- Preregister analytical plans to prevent post-hoc rationalization
- Determine statistical power requirements and sample size needs
Phase 2: Data Collection and Quality Assessment
- Implement comprehensive data profiling examining distributional characteristics
- Identify missing value patterns and mechanisms
- Detect outlier concentrations and anomalies
- Assess correlation structures between variables
Phase 3: Exploratory Analysis and Preprocessing
- Perform descriptive statistical analysis (central tendency, variability measures)
- Conduct distribution analysis to identify patterns, skewness, and outliers
- Implement feature engineering to create biologically informative variables
- Apply appropriate normalization and transformation techniques
Phase 4: Model Development and Validation
- Compare multiple analytical approaches using cross-validation
- Apply ensemble methods combining approaches for improved robustness
- Utilize appropriate performance metrics (AUC, precision, recall, F1-score)
- Implement interpretation techniques to identify important predictive features
Phase 5: Implementation and Monitoring
- Deploy models into production with automated pipeline handling
- Monitor performance and detect concept drift
- Establish feedback mechanisms for continuous model refinement
- Maintain version control and documentation for reproducibility
Visualization Best Practices for Liquid Biopsy Data
Effective data visualization is essential for interpreting complex liquid biopsy results and communicating findings to diverse stakeholders. The following principles ensure clarity and impact [86]:
Color Selection Guidelines:
- Use qualitative palettes with distinct colors for categorical variables (e.g., cancer subtypes)
- Implement sequential palettes with gradient saturations of a single color for ordered numeric values (e.g., variant allele frequencies)
- Apply diverging palettes with two contrasting colors and neutral center for spectrum-based data (e.g., gene expression changes)
- Limit to 7 or fewer colors in a single visualization to avoid cognitive overload
- Ensure sufficient contrast for color vision deficiencies by avoiding red-green combinations
Visualization Types for Liquid Biopsy Data:
- Waterfall plots for displaying mutation profiles across patient cohorts
- Longitudinal line charts for tracking ctDNA dynamics during treatment
- Heatmaps for visualizing multi-omic patterns across patient groups
- Violin plots for comparing distribution characteristics of biomarker levels
- Kaplan-Meier curves for illustrating survival associations
Diagram 2: Bioinformatic workflow from raw data to clinical insights.
Implementation Challenges and Future Directions
Despite significant advances, several challenges remain in the widespread clinical implementation of AI-enhanced liquid biopsy analysis. Addressing these limitations is crucial for translating technological potential into improved patient outcomes.
Data Quality and Standardization:
- Pre-analytical variables significantly impact liquid biopsy results, requiring rigorous standardization of collection, processing, and storage protocols [36]
- Batch effects and technical artifacts can confound biological signals, necessitating careful experimental design and normalization approaches
- Limited annotated datasets, particularly for rare cancer types or early-stage disease, constrain model training and validation [80]
Analytical Validation:
- Determining appropriate limits of detection and quantification for low-frequency variants
- Establishing reproducibility across laboratories and platforms
- Validating clinical utility through prospective randomized trials
Regulatory and Ethical Considerations:
- Evolving regulatory frameworks for AI-based medical devices require careful navigation [81]
- Algorithmic transparency and interpretability demands (explainable AI) are essential for clinical adoption [81]
- Data privacy concerns, particularly with multimodal approaches integrating sensitive health information [81]
Future Directions: The field is rapidly evolving toward more sophisticated and integrated approaches. Multimodal Artificial Intelligence (MMAI) will continue to mature, combining liquid biopsy data with radiomics, digital pathology, and clinical records for comprehensive patient assessment [82]. Quantum machine learning may offer advantages for specific optimization problems in biomarker discovery, though currently limited to specialized applications [83]. Federated learning approaches will enable model training across institutions while preserving data privacy [82]. Longitudinal monitoring algorithms will increasingly focus on tracking tumor evolution and predicting resistance mechanisms before they manifest clinically.
As these technologies mature, the integration of AI-enhanced liquid biopsy into routine cancer care will enable more dynamic, personalized, and preemptive management strategies, ultimately improving early detection and treatment outcomes across the oncology spectrum.
Analytical Validation, Clinical Utility, and Comparative Performance Assessment
Liquid biopsy has emerged as a transformative tool in cancer monitoring, providing a minimally invasive method for detecting and tracking tumor dynamics through the analysis of circulating biomarkers such as circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), and extracellular vesicles (EVs) [71] [42]. Unlike traditional tissue biopsies, liquid biopsies enable real-time snapshots of tumor burden and molecular evolution, making them particularly valuable for assessing therapy response and resistance mechanisms [71]. The analytical validation of these sophisticated assays is paramount to ensuring their reliability and clinical utility. This document outlines the core frameworks for validating key analytical performance characteristics—Limit of Detection (LOD), reproducibility, and precision—within the context of liquid biopsy workflows for cancer research and drug development.
Core Concepts and Definitions
The Limits of Detection and Quantitation
Sensitive detection of low-abundance biomarkers is a central challenge in liquid biopsy. The following concepts are crucial for defining an assay's detection capability [87] [88]:
- Limit of Blank (LoB): The highest apparent analyte concentration expected to be found when replicates of a blank sample containing no analyte are tested. It is defined as LoB = mean¬blank + 1.645(SDblank), assuming a Gaussian distribution where 95% of blank values fall below this limit [87].
- Limit of Detection (LoD): The lowest analyte concentration likely to be reliably distinguished from the LoB. It is determined using both the measured LoB and test replicates of a sample containing a low concentration of analyte: LoD = LoB + 1.645(SDlow concentration sample). A sample at the LoD concentration should be distinguishable from the blank 95% of the time [87] [88].
- Limit of Quantitation (LoQ): The lowest concentration at which the analyte can not only be reliably detected but also measured with predefined levels of bias and imprecision. The LoQ may be equivalent to the LoD or higher, but it cannot be lower than the LoD [87].
Table 1: Summary of Key Detection Capability Metrics
| Parameter | Sample Type | Key Equation | Definition |
|---|---|---|---|
| Limit of Blank (LoB) | Sample containing no analyte [87] | LoB = mean¬blank + 1.645(SDblank) [87] | Highest concentration expected from a blank sample [87] |
| Limit of Detection (LoD) | Sample with low analyte concentration [87] | LoD = LoB + 1.645(SDlow concentration sample) [87] | Lowest concentration reliably distinguished from LoB [87] [88] |
| Limit of Quantitation (LoQ) | Sample at or above the LoD [87] | LoQ ≥ LoD [87] | Lowest concentration measurable with defined precision and bias [87] |
Precision, Accuracy, and Reproducibility
Understanding the variability and reliability of measurements is fundamental to analytical validation.
- Precision vs. Accuracy: Precision refers to the closeness of agreement between independent measurements obtained under the same conditions (a measure of random error or variability), while accuracy refers to the closeness of a measurement to the true value (often describing systematic error or bias) [89] [90]. A measurement system can be precise but not accurate, accurate but not precise, neither, or both [89].
- Repeatability vs. Reproducibility: These are two key components of precision. Repeatability refers to the variation observed when the same instrument and operator repeat measurements over a short time period. Reproducibility refers to the variation observed among different instruments and operators, and over longer time periods [89] [91]. In a quantitative sense, reproducibility can be defined as the standard deviation for the difference between two measurements from different laboratories [91].
- The Reproducibility Crisis: The broader scientific context has highlighted challenges in reproducing results, underscoring the need for robust validation. This has led to a "credibility revolution" emphasizing improved methods, transparency, and open science practices [92].
Experimental Protocols for Validation
Protocol for Determining LoB and LoD
The following protocol, based on CLSI EP17 guidelines, provides a methodology for establishing the detection capability of a liquid biopsy assay [87] [93].
1. Experimental Design:
- Samples: Obtain a blank sample (matrix without the analyte) and a low-concentration sample (e.g., a dilution of the lowest calibrator near the expected LoD).
- Replication: For a thorough characterization, a manufacturer should test at least 60 replicates of each sample. A laboratory verifying a manufacturer's claims may use 20 replicates [87].
- Scope: Perform measurements over multiple days, using different instrument and reagent lots if possible, to capture inter-assay variability [88].
2. Data Collection and Analysis:
- Analyze all samples following the complete analytical procedure, converting raw signals to concentration values using the assay's calibration curve [93].
- For the blank sample, calculate the mean (mean¬blank) and standard deviation (SDblank).
- For the low-concentration sample, calculate the standard deviation (SDlow concentration sample).
3. Calculation:
- Compute the LoB: LoB = mean¬blank + 1.645(SDblank) [87].
- Compute the provisional LoD: LoD = LoB + 1.645(SDlow concentration sample) [87].
- Verification: Analyze multiple samples with a concentration at the provisional LoD. If more than 5% of the results fall below the LoB, the LoD must be re-estimated using a sample of higher concentration [87].
Protocol for Determining LoQ
The LoQ is the point at which quantitative results become reliable.
1. Define Performance Goals: Establish predefined goals for total error, bias, and imprecision (e.g., a coefficient of variation (CV) of ≤20%) [87] [88].
2. Test Sample Series: Prepare and repeatedly analyze (e.g., n=20) a series of samples with concentrations at and above the verified LoD.
3. Calculate Precision and Bias: For each concentration level, calculate the CV (%) and the percent bias from the known concentration.
4. Establish LoQ: The LoQ is the lowest concentration where the predefined goals for both precision and bias are met [87]. This can be identified using methods like CV Profiling from calibration curve data [88].
Protocol for Assessing Precision (Repeatability and Reproducibility)
A nested experimental design is used to quantify different components of variability.
1. Experimental Design:
- Select samples representing low, medium, and high analyte concentrations (e.g., mutant allele frequency in ctDNA).
- Have two or more analysts perform the test on two or more instruments over multiple days (e.g., 2 analysts × 2 instruments × 5 days).
- Each operator should analyze each sample in duplicate or triplicate per run.
2. Data Analysis and Variance Component Estimation:
- Analyze data using a nested Analysis of Variance (ANOVA) model.
- The model will partition the total variance into components representing:
- Between-day variance
- Between-operator variance
- Between-instrument variance
- Within-run (repeatability) variance
3. Calculation of Precision Metrics:
- Repeatability Standard Deviation (S_r): Calculated from the within-run variance component.
- Reproducibility Standard Deviation (S_R): Calculated as the square root of the sum of all variance components [89] [91].
Diagram 1: Precision assessment workflow.
Application in Liquid Biopsy Workflows
Unique Challenges and Considerations
Liquid biopsy presents specific challenges that directly impact validation strategies [71] [42]:
- Low Analytic Concentration: The abundance of key biomarkers like CTCs and ctDNA is often very low, especially in early-stage cancer or minimal residual disease. A typical challenge is finding 1 CTC per 1 million leukocytes, and ctDNA can constitute only 0.1–1.0% of total cell-free DNA [71] [42]. This places a premium on establishing sensitive and robust LoDs.
- Sample Matrix Effects: Blood collection tubes, plasma preparation protocols, and sample storage conditions can significantly impact analyte stability and recovery. For instance, CTCs have a short half-life in circulation (<1–2.5 hours), and ctDNA fragments are shorter than non-tumor cfDNA [71] [42]. Validation must be performed in the intended sample matrix.
- Tumor Heterogeneity: A single liquid biopsy aims to capture the spatial and temporal heterogeneity of a tumor. Assays must be validated to ensure they can detect all clinically relevant subclones without bias [71].
Research Reagent Solutions for Liquid Biopsy
The following table details essential materials and technologies used in the liquid biopsy field.
Table 2: Key Research Reagent Solutions for Liquid Biopsy
| Item/Technology | Function | Example Application in Liquid Biopsy |
|---|---|---|
| CellSearch System [71] | Immunomagnetic CTC isolation and enumeration | FDA-cleared for prognostic CTC counting in metastatic breast, prostate, and colorectal cancers [71] |
| EpCAM Antibodies [71] | Positive selection of epithelial-derived CTCs | Core capture antibody in systems like CellSearch; may miss EpCAM-low CTCs [71] |
| Microfluidic Devices (e.g., CTC-iChip) [71] | Size-based or affinity-based CTC isolation | EpCAM-independent CTC capture, enabling isolation of mesenchymal CTCs [71] |
| BEAMing Technology [42] | Digital PCR-based mutation detection in ctDNA | Highly sensitive method for detecting hotspot mutations (e.g., KRAS, TP53) in plasma [42] |
| Low-Pass Whole-Genome Sequencing (lpWGS) [71] | Detection of genome-wide copy number alterations | Assessing genomic instability in CTCs or ctDNA from prostate cancer [71] |
Diagram 2: Liquid biopsy core workflow.
Rigorous analytical validation is the cornerstone of generating reliable and meaningful data from liquid biopsy assays. By systematically determining the Limit of Detection (LoD), Limit of Quantitation (LoQ), and components of precision (repeatability and reproducibility), researchers can establish the performance boundaries of their methods. This is especially critical in the context of cancer monitoring, where the reliable detection of low-abundance biomarkers like CTCs and ctDNA directly impacts the ability to make accurate clinical and research decisions [71] [42]. Adherence to established guidelines and a thorough understanding of the core concepts outlined in this document will ensure that liquid biopsy fulfills its promise as a robust tool for precision medicine.
Head-to-Head Comparisons of Commercial Liquid Biopsy Assays
Liquid biopsy has emerged as a transformative, minimally invasive tool in precision oncology, enabling real-time characterization of tumor dynamics through the analysis of circulating tumor DNA (ctDNA) and other biomarkers found in bodily fluids [42] [94]. Unlike traditional tissue biopsies, which are limited by sampling constraints and inability to capture tumor heterogeneity, liquid biopsies offer a comprehensive view of the entire tumor burden and facilitate repeated sampling for continuous monitoring of treatment response and disease progression [94] [32].
The clinical utility of liquid biopsy spans multiple applications, including cancer screening, therapy selection, minimal residual disease (MRD) detection, and early relapse prediction [94] [20]. As the field rapidly evolves, numerous commercial liquid biopsy assays have been developed, each with varying technical capabilities and clinical applications. This creates an urgent need for rigorous head-to-head comparisons to guide researchers and clinicians in selecting the most appropriate assays for specific research contexts.
This application note provides a systematic comparison of commercial liquid biopsy assays, detailing performance metrics from recent validation studies and offering standardized protocols for conducting comparative analyses in cancer research settings.
Performance Comparison of Commercial Assays
Comprehensive Genomic Profiling Assays
Table 1: Performance Comparison of CGP Liquid Biopsy Assays
| Assay Name | Variant Types Detected | Genes Covered | Limit of Detection (LOD) | Key Performance Metrics | Reference Assays |
|---|---|---|---|---|---|
| Northstar Select | SNV/Indels, CNVs, Fusions, MSI | 84 | 0.15% VAF (SNV/Indels); 2.11 copies (amplifications); 1.80 copies (losses); 0.30% (fusions) | 51% more pathogenic SNV/indels; 109% more CNVs; 45% fewer null reports | Six on-market CGP assays from four CLIA/CAP labs [95] |
| Guardant360 CDx | SNV, Indels, CNVs, Fusions | N/A | N/A | FDA-approved as companion diagnostic for ESR1 mutations in breast cancer | N/A [96] |
The recent prospective head-to-head comparison study demonstrated the superior sensitivity of the Northstar Select assay across multiple variant classes [95] [97]. Notably, the majority (91%) of additional clinically actionable SNV/indels identified by Northstar Select were detected below 0.5% variant allele frequency (VAF), highlighting its enhanced capability to detect low-frequency variants in low-shedding tumors [95]. This increased sensitivity directly addresses a key challenge in liquid biopsy testing and has significant implications for clinical decision-making by enabling identification of more actionable genomic alterations.
Minimal Residual Disease and Monitoring Assays
Table 2: Performance Comparison of MRD Liquid Biopsy Assays
| Assay Name | Technology | Reported Sensitivity | Clinical Utility | Cancer Types Validated |
|---|---|---|---|---|
| Signatera (Natera) | Tumor-informed, custom | N/A | MRD detection, recurrence monitoring | Colorectal, Breast, Bladder [94] |
| Guardant Reveal | Tumor-naive | N/A | MRD detection, recurrence monitoring | Various [94] |
| NeXT Personal | Tumor-informed, WGS-based | Detects ctDNA down to 10 ppm | Predicts outcomes in neoadjuvant setting | EGFR-mutated NSCLC [96] |
| FoundationOne MRD | Tissue-informed WGS | Detects ctDNA down to 10 ppm | Research use only | Early to late-stage cancers [96] |
Longitudinal liquid biopsy testing has demonstrated significant clinical value in monitoring treatment response and predicting recurrence. In a real-world analysis of 30 patients with diverse solid tumors, serial ctDNA testing meaningfully influenced clinical decisions across four key categories: treatment escalation (23% of cases), treatment de-escalation (13% of cases), response monitoring (43% of cases), and early relapse prediction (20% of cases) [94]. The ability to dynamically monitor tumor burden through ctDNA analysis provides a critical tool for guiding personalized treatment strategies.
Figure 1: Workflow for conducting head-to-head comparisons of liquid biopsy assays, showing the sequential phases from study design through experimental execution to data analysis.
Experimental Protocols for Assay Comparison
Sample Collection and Processing Protocol
Materials:
- Kâ‚‚EDTA or Streck Cell-Free DNA blood collection tubes
- Centrifuge capable of 1600-3000 × g
- DNA extraction kit (e.g., QIAamp Circulating Nucleic Acid Kit)
- Qubit fluorometer or equivalent for DNA quantification
- Agilent Bioanalyzer or TapeStation for DNA quality control
Procedure:
- Blood Collection: Draw 10-20 mL of whole blood into appropriate collection tubes. Invert gently 8-10 times to mix.
- Plasma Separation: Centrifuge at 1600-3000 × g for 10-20 minutes at 4°C within 2 hours of collection.
- Plasma Transfer: Carefully transfer supernatant plasma to a sterile tube without disturbing the buffy coat.
- Secondary Centrifugation: Centrifuge plasma at 16,000 × g for 10 minutes to remove residual cells.
- cfDNA Extraction: Extract cfDNA from 2-10 mL plasma using specialized circulating nucleic acid kits according to manufacturer's instructions.
- Quality Control: Quantify DNA yield using fluorometric methods and assess fragment size distribution using microcapillary electrophoresis.
Critical Step Notes: Process plasma within 6 hours of collection when using Kâ‚‚EDTA tubes. Streck tubes allow for longer processing windows (up to 14 days) but should be processed consistently across compared assays. Avoid repeated freeze-thaw cycles of plasma and extracted cfDNA [42] [71].
Analytical Validation Protocol for Sensitivity Assessment
Materials:
- Commercial reference standards with known mutation VAFs
- Digital PCR system for orthogonal validation
- Next-generation sequencing platform
- Bioinformatics pipelines for variant calling
Procedure:
- Reference Material Preparation: Dilute commercial reference standards to create a series of samples with VAFs ranging from 2.0% to 0.1%.
- Parallel Processing: Process identical reference samples using each assay being compared according to manufacturer's protocols.
- Variant Calling: Perform bioinformatic analysis using default parameters for each platform.
- Orthogonal Validation: Confirm variant calls using digital PCR for a subset of variants.
- Sensitivity Calculation: Calculate detection sensitivity at each VAF level using the formula: (True Positives / (True Positives + False Negatives)) × 100.
- Limit of Detection Determination: Identify the lowest VAF at which ≥95% of expected variants are detected for each assay.
Critical Step Notes: Use identical input DNA masses across all compared assays. Include a minimum of three replicates at each VAF level to assess reproducibility. Blinding technicians to expected variant status reduces bias [95] [97].
Clinical Validation Protocol Using Patient Samples
Materials:
- Matched tumor tissue and blood samples from cancer patients
- Institutional Review Board-approved study protocol
- Clinical data collection forms
- Radiological imaging data for response assessment
Procedure:
- Patient Recruitment: Enroll patients with confirmed cancer diagnoses across multiple stages and cancer types.
- Sample Collection: Collect matched tissue biopsies and blood samples at predetermined timepoints (baseline, during treatment, at progression).
- Blinded Testing: Process liquid biopsy samples using each assay in a blinded manner.
- Data Collection: Document all pathogenic alterations identified by each platform.
- Actionable Alteration Assessment: Categorize alterations based on clinical actionability using established guidelines.
- Clinical Correlation: Compare assay results with clinical outcomes including treatment response and progression-free survival.
Critical Step Notes: Ensure adequate sample size for statistical power (typically 100+ patients). Stratify patients by cancer type and stage. Define clinical endpoints prospectively [95] [94].
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions for Liquid Biopsy Assays
| Category | Specific Product Examples | Function & Application |
|---|---|---|
| Blood Collection Tubes | Kâ‚‚EDTA tubes; Streck Cell-Free DNA BCT; PAXgene Blood cDNA tubes | Preserve blood samples for plasma separation and prevent genomic DNA contamination [42] |
| Nucleic Acid Extraction | QIAamp Circulating Nucleic Acid Kit; MagMax Cell-Free DNA Isolation Kit | Isolate high-quality cfDNA from plasma samples [71] |
| Library Preparation | AVENIO ctDNA Library Prep Kit; QIAseq Targeted DNA Panel; xGen cfDNA & FFPE DNA Library Prep | Prepare sequencing libraries optimized for fragmented cfDNA [95] |
| Target Enrichment | IDT xGen Lockdown Probes; Twist Human Core Exome; Agilent SureSelectXT | Capture genomic regions of interest for targeted sequencing [95] |
| Reference Standards | Seraseq ctDNA Reference Materials; Horizon Multiplex I cfDNA Reference Standard | Validate assay performance and determine sensitivity [95] |
| Quality Control | Agilent Bioanalyzer High Sensitivity DNA Kit; Qubit dsDNA HS Assay Kit; TapeStation Genomic DNA ScreenTape | Assess DNA quality, quantity, and fragment size distribution [71] |
Figure 2: Clinical decision pathways in liquid biopsy testing, showing how ctDNA results guide four distinct clinical actions: treatment escalation, treatment de-escalation, response monitoring, and early relapse prediction.
Head-to-head comparisons of commercial liquid biopsy assays reveal significant differences in analytical sensitivity and clinical utility. The recent validation of the Northstar Select assay demonstrates how technological advancements can address key challenges in liquid biopsy testing, particularly for detecting variants in low-shedding tumors [95]. The superior sensitivity of this assay (51% more pathogenic SNV/indels and 109% more CNVs compared to existing commercial tests) highlights the rapid pace of innovation in this field.
For researchers designing studies involving liquid biopsy, careful consideration of assay sensitivity, variant coverage, and clinical validation status is essential. The protocols provided in this application note offer a standardized framework for conducting rigorous comparisons of liquid biopsy assays, enabling researchers to select the most appropriate platforms for their specific cancer monitoring research needs. As the field continues to evolve, ongoing comparative studies will be essential for identifying optimal applications of each technology in the precision oncology workflow.
Within the framework of liquid biopsy workflows for cancer monitoring, understanding the concordance between traditional tissue biopsy and plasma-based genomic profiling is paramount for researchers and drug development professionals. Next-generation sequencing (NGS) of solid tumor tissue is the established standard for identifying targetable genomic variants. However, the emergence of circulating tumor DNA (ctDNA) analysis presents a less invasive alternative that can overcome challenges such as tumor heterogeneity and the inaccessibility of tissue samples [98]. This application note synthesizes recent clinical evidence to evaluate the feasibility and limitations of using plasma-based NGS, providing detailed protocols and data analysis to guide its application in oncology research.
Quantitative Concordance Data
Clinical studies directly comparing matched tissue and plasma samples reveal a generally high concordance, particularly for specific mutation types and when ctDNA is detectable.
Table 1: Patient-Level and Variant-Level Concordance from Clinical Studies
| Metric | Study 1 (146 Patients) [98] | Study 2 (190 Patients) [99] |
|---|---|---|
| Patient Cohort | Chinese lung cancer patients | Non-small cell lung cancer (NSCLC) patients |
| Patients with ≥1 Concordant Variant | >80% | 81 patients (42.6%) |
| Overall Concordance Rate | - | 78.9% (150/190 patients) |
| Concordance (ctDNA+ subgroup) | - | 91.2% (125/137 patients) |
| Sensitivity of Plasma-NGS | 53.9% (all variants) | 71.9% (entire cohort); 93.5% (ctDNA+ subgroup) |
| Positive Predictive Value (PPV) | 84.6% | - |
Table 2: Sensitivity of Plasma-Based NGS for Key Driver Alterations in NSCLC [98]
| Genomic Alteration | Sensitivity in Plasma |
|---|---|
| EGFR exon 19 deletion (19del) | 90% |
| EGFR p.S768I | 100% |
| ALK fusion | 85.7% |
| RET fusion | 100% |
| KRAS p.G12C | 85.7% |
Table 3: Comparison of Alteration Detection by Type [99]
| Alteration Type | Detection Capability in Plasma |
|---|---|
| Single Nucleotide Variants (SNVs) & Insertions/Deletions (Indels) | High sensitivity |
| Copy Number Variations (CNVs) | Significantly less capable |
| Gene Fusions | Significantly less capable |
Experimental Protocols for Concordance Studies
Sample Collection and Processing
Materials:
- Patient Samples: Matched formalin-fixed paraffin-embedded (FFPE) tissue blocks and peripheral blood samples.
- Blood Collection Tubes: EDTA or specialized cell-free DNA blood collection tubes.
- Centrifuges: Capable of 2000 g and 16,000 g at 4°C.
- Extraction Kits: QIAamp Circulating Nucleic Acid Kit (for cfDNA) and QIAamp DNA FFPE Tissue Kit (for tissue DNA) [98].
Procedure:
- Sample Collection: Collect peripheral blood (e.g., 10 mL) and store at 4°C immediately after draw.
- Plasma Separation: Centrifuge blood at 2000 g for 10 min at 4°C. Transfer the supernatant (plasma) to a new tube and perform a second centrifugation at 16,000 g for 10 min at 4°C to remove residual cells.
- Storage: Aliquot the purified plasma and store at -80°C until DNA extraction.
- DNA Extraction: Extract cfDNA from plasma using the QIAamp Circulating Nucleic Acid Kit. Extract genomic DNA from FFPE tissue sections using the QIAamp DNA FFPE Tissue Kit, following the manufacturer's instructions.
- Quantification: Measure DNA concentration using a fluorometer (e.g., Qubit 2.0 with dsDNA HS Assay Kit) [98].
Library Preparation and Target Sequencing
Materials:
- Fragmentation Instrument: Covaris M220 focused ultrasonicator.
- Library Prep Reagents: End repair, phosphorylation, dA addition, and adaptor ligation modules.
- Selection Beads: Agencourt AMPure XP beads.
- Target Capture Panel: A commercially available 168-gene cancer-related panel (e.g., from Burning Rock Biotech) [98].
- Sequencing Platform: Illumina NextSeq 500 [98].
Procedure:
- Fragmentation: Fragment 20-80 ng of tissue DNA or cfDNA using the Covaris M220.
- Library Construction: Perform end repair, phosphorylation, dA-tailing, and adaptor ligation to create sequencing libraries.
- Size Selection: Use AMPure XP beads to select DNA fragments between 200-400 bp.
- Target Enrichment: Hybridize libraries with biotinylated probes targeting the 168-gene panel. Capture hybridized fragments using streptavidin-coated magnetic beads and amplify via PCR.
- Sequencing: Pool indexed libraries and sequence on an Illumina NextSeq 500 using paired-end runs [98].
Bioinformatic Analysis
Software/Tools:
- Alignment: Burrows-Wheeler Aligner (BWA v.0.7.10) for mapping reads to the human reference genome (hg19).
- Variant Calling: Genome Analysis Toolkit (GATK v.3.2) and VarScan (v.2.4.3).
- Fusion Detection: Factera (v.1.4.3).
- Annotation: ANNOVAR and SnpEff (v.3.6) [98].
Procedure:
- Data Processing: Map raw sequencing reads to the reference genome.
- Variant Identification: Call SNVs/Indels and copy number variations (CNVs) using the specified tools. For gene fusions, use specialized translocation analysis software.
- Filtering: Filter out variants with a population frequency >0.1% (using databases like 1000 Genomes and dbSNP) to remove common polymorphisms.
- Thresholding: Apply the following allele frequency (AF) thresholds: AF ≥ 1% for tissue variants and AF ≥ 0.1% for plasma variants. Consider CNV amplification for genes with copy number >2.25 [98].
- Concordance Analysis: Compare the list of variants identified in matched tissue and plasma samples to calculate patient-level and variant-level concordance, sensitivity, and positive predictive value.
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Reagents for NGS-Based Concordance Studies
| Reagent / Kit | Function | Application Note |
|---|---|---|
| QIAamp Circulating Nucleic Acid Kit | Extraction of high-quality, inhibitor-free cell-free DNA from plasma. | Critical for obtaining sufficient yields of fragmented cfDNA for low-AF variant detection [98]. |
| QIAamp DNA FFPE Tissue Kit | Extraction of genomic DNA from formalin-fixed, paraffin-embedded tissue. | Optimized to overcome DNA fragmentation and cross-linking inherent in FFPE samples [98]. |
| Agencourt AMPure XP Beads | Solid-phase reversible immobilization (SPRI) for DNA size selection and purification. | Used to select library fragments of 200-400 bp, removing primer dimers and large contaminants [98]. |
| 168-Gene Cancer Panel | Targeted enrichment of genomic regions of interest for sequencing. | Provides a comprehensive view of actionable mutations and fusions in lung cancer while maintaining cost-effectiveness [98] [99]. |
| DAB Chromogen/Substrate Kit | Chromogenic detection for immunohistochemical staining. | Used in companion IHC assays to validate protein expression (e.g., ALK, ROS1) from tissue sections [100]. |
| NBT/BCIP Substrate | Chromogenic substrate for alkaline phosphatase (AP), yielding a black-purple precipitate. | An alternative chromogen for IHC or western blot detection when using AP-conjugated antibodies [101] [102]. |
Emerging Biomarkers and Future Directions
The liquid biopsy field is rapidly evolving beyond ctDNA. Circular RNAs (circRNAs) are a promising class of biomarkers due to their closed-loop structure, which confers high stability in bodily fluids [103]. Their role in mediating drug resistance through mechanisms like microRNA sponging makes them particularly attractive for longitudinal therapy monitoring.
Table 5: Exemplary circRNAs Implicated in Cancer Drug Resistance [103]
| CircRNA | Cancer Type | Associated Resistance | Proposed Mechanism |
|---|---|---|---|
| circ_0001946 | NSCLC | Gefitinib (EGFR-TKI) | Activates STAT6/PI3K/AKT pathway via miR-135a-5p sponging |
| circRNA_102231 | NSCLC | Gefitinib (EGFR-TKI) | Acts as a sponge for miR-130a-3p |
| circHIPK3 | Colorectal, Lung | 5-FU, Cisplatin | Sponges tumor-suppressor miRNAs like miR-124 |
| circ-PVT1 | Gastric Cancer | Paclitaxel | Modulates epithelial-mesenchymal transition (EMT) |
| circAKT3 | Glioblastoma | Temozolomide | Promotes stemness via PI3K/AKT pathway |
Evidence from concordance studies supports plasma-based NGS as a reliable tool for detecting key driver mutations in lung cancer, especially SNVs and Indels like EGFR 19del and KRAS G12C [98]. However, tissue-NGS remains the more comprehensive method, showing superior sensitivity for CNVs and fusions [99]. The optimal research strategy, therefore, involves a complementary approach: using liquid biopsy for non-invasive, longitudinal monitoring of disease evolution and therapy response, while relying on tissue biopsy for initial comprehensive genotyping and when liquid biopsy results are negative despite clinical evidence of disease. The integration of novel biomarkers like circRNAs further promises to enhance the utility of liquid biopsy workflows in cancer monitoring and drug development research.
Circulating tumor DNA (ctDNA) has emerged as a transformative biomarker in oncology, enabling non-invasive assessment of tumor burden, genetic heterogeneity, and therapeutic response [42] [2]. As a component of cell-free DNA (cfDNA) shed into the bloodstream by tumor cells, ctDNA constitutes approximately 0.1-1.0% of total cfDNA in cancer patients and carries tumor-specific genetic and epigenetic alterations [42] [2]. The analysis of ctDNA dynamics—changes in concentration, variant allele frequency (VAF), and molecular profile over time—provides a powerful approach for monitoring treatment efficacy, detecting minimal residual disease (MRD), and identifying emerging resistance mechanisms [104] [105]. This protocol outlines standardized methodologies for correlating ctDNA dynamics with treatment outcomes across various cancer types, with specific applications in non-small cell lung cancer (NSCLC), colorectal cancer (CRC), pancreatic ductal adenocarcinoma (PDAC), and breast cancer.
Quantitative Evidence: ctDNA Dynamics and Clinical Outcomes
Table 1: Key Clinical Studies on ctDNA Dynamics and Treatment Outcomes
| Cancer Type | Study Design | ctDNA Assessment Method | Key Findings | Clinical Correlation |
|---|---|---|---|---|
| Metastatic Colorectal Cancer | Systematic review & meta-analysis (56 studies, n=3,735) [105] | Various NGS & PCR-based methods | ctDNA increase during therapy: HR 2.44 for PFS (95% CI: 2.02-2.95); HR 2.53 for OS (95% CI: 2.01-3.18) | Strong prognostic value for progression and survival |
| Stage II Colon Cancer | DYNAMIC Trial (5-year follow-up) [106] | Tumor-informed multiplex PCR (29 mutations/patient) | ctDNA clearance post-chemotherapy: 97% 5-year RFS vs. 0% for ctDNA persistence (P<0.001) | Predictive of long-term treatment benefit |
| Colorectal Cancer | Systematic review & meta-analysis (65 studies) [107] | Various NGS & PCR-based methods | Post-treatment ctDNA detection: HR 8.92 for RFS (95% CI: 6.02-13.22); HR 3.05 for OS (95% CI: 1.72-5.41) | Powerful prognostic prediction after completed treatment |
| Metastatic PDAC [108] | Monocentric cohort (n=71) | ddPCR targeting methylated markers (HOXD8, POU4F1) | Correlation between ctDNA quantity and liver metastases tumor volume (Spearman's Ï=0.500, p<0.001) | ctDNA level reflects metastatic burden |
| NSCLC [104] | Review of multiple studies | NGS-based & digital PCR methods | ctDNA levels fluctuate with treatment response; higher levels linked to poorer prognosis | Correlation with tumor burden and treatment response |
Table 2: ctDNA Detection Technologies and Performance Characteristics
| Technology Platform | Detection Sensitivity | Advantages | Limitations | Optimal Applications |
|---|---|---|---|---|
| Next-Generation Sequencing (NGS) [104] [2] | ~0.1% VAF (standard); <0.01% VAF (ultrasensitive) | Comprehensive genomic profiling; detects multiple mutation types | Longer turnaround time; higher cost; requires bioinformatics | Tumor genotyping; MRD detection; resistance monitoring |
| Digital PCR (ddPCR) [108] [2] | ~0.1% VAF | Absolute quantification; rapid turnaround; high sensitivity for known variants | Limited to pre-specified mutations; cannot detect novel alterations | Tracking specific mutations; treatment monitoring |
| Structural Variant-Based Assays [2] | <0.01% VAF (parts-per-million sensitivity) | Ultra-sensitive; tumor-specific rearrangements; low background noise | Requires personalized assay design | MRD detection; early-stage cancer monitoring |
| Methylation-Based Profiling [108] [2] | Varies by platform | Epigenetic information; tumor origin identification; early detection potential | Complex analysis; requires validation | Cancer subtyping; early detection; monitoring |
| Electrochemical Biosensors [2] | Attomolar concentrations | Rapid results (20 mins); point-of-care potential; low cost | Early development stage; limited clinical validation | Future rapid monitoring applications |
Experimental Protocols
Protocol 1: Longitudinal ctDNA Monitoring for Treatment Response Assessment
Purpose: To evaluate therapy effectiveness by tracking quantitative ctDNA changes during treatment.
Materials Required:
- Blood collection tubes (cfDNA-specific stabilization tubes)
- DNA extraction kit (cfDNA-specific)
- Targeted NGS panel or ddPCR assays
- Bioinformatics pipeline for variant calling
- Quantitative calibration standards
Procedure:
- Baseline Sample Collection: Collect 10-20 mL blood in cfDNA stabilization tubes prior to treatment initiation [2]
- Serial Sampling: Collect follow-up samples at predetermined intervals (e.g., every 2-4 weeks during active treatment, then every 3 months during surveillance) [105]
- Sample Processing:
- Centrifuge within 2 hours of collection (1600 × g for 10 min, then 16,000 × g for 10 min)
- Extract cfDNA using silica-membrane columns or magnetic beads
- Quantify cfDNA yield by fluorometry [2]
- ctDNA Analysis:
- For NGS: Prepare libraries with unique molecular identifiers (UMIs)
- Sequence with minimum 10,000x coverage for targeted panels
- For ddPCR: Run reactions in triplicate with negative controls [108]
- Data Analysis:
- Calculate mutant allele frequency (MAF) for tracked variants
- Determine ctDNA concentration (haploid genome equivalents/mL)
- Compare serial measurements to baseline
Interpretation: A >50% decrease in ctDNA concentration after one treatment cycle correlates with radiographic response, while increasing levels suggest progression [105]. Early ctDNA clearance (within first 2 cycles) may predict favorable outcomes [106].
Protocol 2: Post-Treatment Minimal Residual Disease Detection
Purpose: To identify molecular recurrence before radiographic evidence of disease.
Materials Required:
- Tumor tissue (archived FFPE blocks)
- Matched peripheral blood (germline control)
- Ultra-sensitive NGS platform (e.g., PhasED-seq, SV-based assays)
- Personalized assay design capabilities
Procedure:
- Tumor Genotyping:
- Sequence tumor DNA to identify clonal mutations
- Select 16-50 variants for tracking based on clonality and assay compatibility [106]
- Assay Design:
- Design personalized NGS panel or PCR assays for selected variants
- Include UMIs and error suppression methods [2]
- Baseline Collection: Obtain plasma sample 2-4 weeks after completion of definitive therapy
- Longitudinal Monitoring: Collect samples every 3-4 months for 2 years, then every 6 months [107]
- Analysis:
- Use duplex sequencing methods with error correction
- Require ≥2 variant molecules detected for positivity
- Report variant allele frequencies with confidence intervals [2]
Interpretation: MRD positivity (ctDNA detection after treatment) associates with significantly worse RFS (HR 8.92) and OS (HR 3.05) in colorectal cancer [107]. In stage II colon cancer, ctDNA clearance after adjuvant chemotherapy predicts 97% 5-year RFS versus 0% for persistent ctDNA [106].
Protocol 3: Resistance Mutation Monitoring
Purpose: To detect emerging resistance mutations during targeted therapy.
Materials Required:
- Targeted NGS panel covering known resistance mechanisms
- ddPCR assays for common resistance mutations (e.g., EGFR T790M, KRAS G12C)
- Plasma processing equipment
- Bioinformatics tools for subclonal variant detection
Procedure:
- Baseline Establishment: Document sensitizing mutations prior to targeted therapy initiation
- Monitoring Schedule: Collect plasma samples every 4-8 weeks during treatment
- Analysis:
- Use NGS with high sequencing depth (>20,000x) for resistance mutation detection
- Employ ddPCR for ultrasensitive quantification of known resistance mutations [104]
- Variant Reporting:
- Report VAF of resistance mutations relative to original driver mutation
- Track subclonal dynamics over time
Interpretation: Emerging resistance mutations in ctDNA often appear weeks to months before radiographic progression [2]. In EGFR-mutant NSCLC, T790M detection in ctDNA can guide switching to third-generation EGFR inhibitors without repeat tissue biopsy [2].
Visualizing ctDNA Dynamics and Clinical Applications
Figure 1: Clinical Decision Pathways Based on ctDNA Dynamics
Figure 2: ctDNA Analysis Workflow from Sample to Result
Research Reagent Solutions
Table 3: Essential Materials for ctDNA Analysis
| Category | Specific Products/Technologies | Key Features | Application Notes |
|---|---|---|---|
| Blood Collection Systems | cfDNA BCT Streck tubes, PAXgene Blood ccfDNA tubes | Cell-stabilizing chemistry, prevents background DNA release | Critical for pre-analytical quality; enables sample stability during transport |
| Nucleic Acid Extraction | QIAamp Circulating Nucleic Acid Kit, MagMAX Cell-Free DNA Isolation Kit | High recovery of short fragments, removal of inhibitors | Aim for >80% recovery of 100-150bp fragments; quantify by fluorometry |
| Library Preparation | AVENIO ctDNA Library Prep Kits, QIAseq Ultra Panels, IDT xGen cfDNA | UMI incorporation, low input compatibility, minimal bias | Unique Molecular Identifiers (UMIs) essential for error correction |
| Target Enrichment | Hybrid capture panels (Illumina, Roche), Amplicon panels (IDT, Archer) | Comprehensive coverage, uniform coverage | Hybrid capture offers broader coverage; amplicon provides deeper sequencing |
| Sequencing Platforms | Illumina NextSeq 550Dx, NovaSeq, Ion Torrent Genexus | Clinical validation, automated workflow, fast turnaround | Minimum 10,000x coverage recommended for variant detection |
| Digital PCR Systems | Bio-Rad ddPCR, Qiagen QIAcuity, Thermo Fisher QuantStudio | Absolute quantification, high sensitivity for known variants | Ideal for tracking specific mutations during treatment |
| Bioinformatics Tools | Illumina DRAGEN, Archer Analysis, In-house pipelines | UMI processing, error suppression, variant annotation | Must include error correction algorithms for low VAF detection |
The correlation between ctDNA dynamics and treatment outcomes represents a paradigm shift in cancer monitoring, offering real-time, non-invasive assessment of therapeutic response and disease evolution. The protocols outlined herein provide standardized approaches for implementing ctDNA monitoring in clinical research settings, with robust evidence supporting its prognostic and predictive value across multiple cancer types. As ctDNA analysis technologies continue to advance toward attomolar sensitivity and point-of-care applications, the integration of liquid biopsy into cancer management protocols promises to enhance personalized treatment strategies, enable earlier intervention, and ultimately improve patient outcomes. Future directions include standardization of assay performance, validation of interventional trials based on ctDNA monitoring, and development of integrated artificial intelligence platforms for comprehensive biomarker interpretation.
Conclusion
Liquid biopsy has matured into an indispensable tool for cancer monitoring, offering a non-invasive window into tumor dynamics that is transforming precision oncology and drug development. The integration of multiple biomarker classes—from ctDNA and CTCs to epigenetic markers—coupled with ultrasensitive detection technologies, enables comprehensive genomic profiling, MRD detection, and real-time therapy response monitoring. Despite persistent challenges in standardization and sensitivity for low-shedding tumors, recent advancements in AI integration and multimodal approaches are rapidly addressing these limitations. The growing body of clinical evidence, including large-scale studies like NCI-MATCH, demonstrates strong concordance with tissue biopsy and validates the utility of liquid biopsy for patient stratification and clinical trial endpoints. Future directions will focus on establishing ctDNA as a validated surrogate endpoint, expanding multi-cancer early detection applications, and further harmonizing methodologies to fully realize the potential of liquid biopsy in delivering personalized, dynamically adaptive cancer care.
References
- 1. Circulating tumor DNA to monitor treatment response in ... [https://www.nature.com/articles/s41698-025-00876-y]
- 2. Monitoring and Assessment of Circulating Tumor DNA in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12550271/]
- 3. International expert consensus on the clinical integration of ... [https://www.sciencedirect.com/science/article/abs/pii/S0959804925009360]
- 4. Circulating tumor cells as predictive biomarkers in the risk ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12571075/]
- 5. Extracellular vesicles as biomarkers in cancer diagnosis ... [https://pubmed.ncbi.nlm.nih.gov/41248958/]
- 6. Improvement of the sensitivity of circulating tumor DNA- ... [https://www.explorationpub.com/Journals/etat/Article/1002333]
- 7. Advances in CTC and ctDNA detection techniques [https://breast-cancer-research.biomedcentral.com/articles/10.1186/s13058-025-02024-7]
- 8. Circulating tumor DNA predicts prognosis at different time ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12507613/]
- 9. MA06.04 Improving Preoperative ctDNA Detection in ... [https://www.sciencedirect.com/science/article/pii/S155608642501192X]
- 10. Molecular counting enables accurate and precise ... [https://www.nature.com/articles/s41598-025-90013-3]
- 11. Circulating tumor cells: overcoming challenges of detecting ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12123222/]
- 12. Detection of circulating tumor cells: opportunities and challenges [https://biomarkerres.biomedcentral.com/articles/10.1186/s40364-022-00403-2]
- 13. Clinical applications of circulating tumor cells in metastasis ... [https://jhoonline.biomedcentral.com/articles/10.1186/s13045-025-01733-y]
- 14. Circulating tumor cell markers for early detection and drug ... [https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2025.1494723/full]
- 15. refining circulating tumor cells enumeration for improved ... [https://pubmed.ncbi.nlm.nih.gov/40968724/]
- 16. Cancer in a Drop: Liquid biopsy insights from AACR 2025 [https://pmc.ncbi.nlm.nih.gov/articles/PMC12223554/]
- 17. Isolation of circulating tumor cells: recent progress and ... [https://link.springer.com/article/10.1007/s44258-024-00044-0]
- 18. The Emergence of Liquid Biopsy and Epigenetic Markers - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC12433903/]
- 19. Epigenetics and CTCs: New biomarkers and impact on ... [https://www.sciencedirect.com/science/article/abs/pii/S1937644824000418]
- 20. a roadmap for DNA methylation biomarkers in liquid biopsies [https://www.nature.com/articles/s41388-025-03624-5]
- 21. Deep generative AI models analyzing circulating orphan ... [https://www.nature.com/articles/s41467-024-53851-9]
- 22. Advancements in DNA methylation technologies and their ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12309559/]
- 23. Guardant Health Expands Tissue-Free Reveal Test to ... [https://investors.guardanthealth.com/press-releases/press-releases/2025/Guardant-Health-Expands-Tissue-Free-Reveal-Test-to-Include-Late-Stage-Therapy-Response-Monitoring/default.aspx]
- 24. Proceedings of cell-free noncoding RNA biomarker studies in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12495990/]
- 25. Special Issue “Circulating Non-Coding RNAs as Diagnostic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12524499/]
- 26. Multi-Biofluid Approaches for cftDNA and cftRNA ... [https://www.mdpi.com/1467-3045/47/9/738]
- 27. Biomarker definitions and their applications - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC5813875/]
- 28. MarkerDB 2.0: a comprehensive molecular biomarker ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11701609/]
- 29. A Comparative Analysis of Biomarker Selection Techniques [https://pmc.ncbi.nlm.nih.gov/articles/PMC3842054/]
- 30. Types of biomarkers and their applications [https://www.abcam.com/en-us/knowledge-center/immunology-and-infectious-disease/types-of-biomarkers-and-their-applications]
- 31. Liquid biopsy – a narrative review with an update on current ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12333414/]
- 32. Liquid biopsy in cancer management: Integrating ... [https://www.sciencedirect.com/science/article/pii/S2352551724000921]
- 33. Preanalytical Errors in Clinical Laboratory Testing at a ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10981510/]
- 34. Evaluation of automatic cell free DNA extraction metrics using different... [https://www.nature.com/articles/s41598-025-03508-4?error=cookies_not_supported&code=70bac897-ac44-46df-90cb-ce411a1cc49e]
- 35. Isolation and Quantification of Plasma Cell-Free DNA ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9601152/]
- 36. From Sampling to Sequencing: A Liquid Biopsy Pre-Analytic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8232701/]
- 37. A novel method for liquid-phase extraction of cell-free DNA ... [https://www.nature.com/articles/s41598-021-98815-x]
- 38. Rapid and Efficient Extraction of Cell-Free DNA Using ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9405790/]
- 39. Evaluation of variability in cell-free DNA extraction ... [https://www.nature.com/articles/s41598-025-06563-z]
- 40. Liquid Biopsy Test: Protocol and Steps [https://www.technologynetworks.com/diagnostics/articles/liquid-biopsy-test-protocol-and-steps-370380]
- 41. Illumina Comprehensive Genomic Profiling Test for Cancer ... [https://www.illumina.com/company/news-center/press-releases/2025/586da3e1-460e-4a24-a018-0d82b7e35c01.html]
- 42. Liquid biopsy in cancer: current status, challenges and ... [https://www.nature.com/articles/s41392-024-02021-w]
- 43. Liquid biopsy in cancer management: Integrating diagnostics ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11743551/]
- 44. Complete Genomics Highlights Clinical Research ... [https://www.completegenomics.com/complete-genomics-highlights-clinical-research-sequencing-solutions/]
- 45. Oncomine Comprehensive Genomic Profiling NGS ... [https://www.thermofisher.com/us/en/home/clinical/preclinical-companion-diagnostic-development/oncomine-oncology/oncomine-cancer-research-panel-workflow.html]
- 46. DNA methylation in breast cancer: early detection and ... [https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-025-06495-2]
- 47. Cell-free DNA fragmentomics: a universal framework for ... [https://pubmed.ncbi.nlm.nih.gov/40977920/]
- 48. The advances of DNA methylation in the liquid biopsy for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12259442/]
- 49. Can a Liquid Biopsy Detect Early-Stage RCC? [https://www.cancernursingtoday.com/post/fragmentomics-liquid-biopsy-accurately-detects-early-rcc]
- 50. Computational Workflow for Genome-Wide DNA ... [https://pubmed.ncbi.nlm.nih.gov/41230550/]
- 51. Minimal Residual Disease Detection: Implications for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12079024/]
- 52. A comprehensive overview of minimal residual disease in ... [https://www.nature.com/articles/s41698-025-00984-9]
- 53. Measurable residual disease monitoring in acute myeloid ... [https://www.sciencedirect.com/science/article/pii/S0037196325000290]
- 54. Liquid Biopsy as a Tool for the Diagnosis, Treatment, and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9456236/]
- 55. Minimal Residual Testing Market [https://bisresearch.com/insights/top-companies-and-breakthroughs-in-minimal-residual-disease-mrd-testing-in-2025]
- 56. Liquid biopsy helps advance oncology drug development [https://www.drugtargetreview.com/article/151342/liquid-biopsy-helps-advance-oncology-drug-development/]
- 57. Clinical Trials - What is holding liquid biopsy back? [https://frontlinegenomics.com/clinical-trials-what-is-holding-liquid-biopsy-back/]
- 58. Cancer Liquid Biopsy Research | NGS to detect tumor ... [https://www.illumina.com/areas-of-interest/cancer/research/applications/liquid-biopsy-research.html]
- 59. Measurement of ctDNA Tumor Fraction Identifies ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11145175/]
- 60. ctDNA Tumor Fraction Matters When Interpreting Liquid ... [https://www.foundationmedicine.com/resources/knowledge-center/ctdna-tumor-fraction-matters-when-interpreting-liquid-biopsy-test-results]
- 61. Can a Liquid Biopsy Detect Circulating Tumor DNA With ... [https://pubmed.ncbi.nlm.nih.gov/38905450/]
- 62. Techniques of using circulating tumor DNA as a liquid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6197739/]
- 63. A Review of Circulating Tumor DNA (ctDNA) and the Liquid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12032849/]
- 64. Novel applications of liquid Biopsy [https://www.sciencedirect.com/science/article/pii/S2950195425000232]
- 65. A spectroscopic liquid for the biopsy of multiple... earlier detection [https://pubmed.ncbi.nlm.nih.gov/37717120/]
- 66. to Liquid - Biopsy Liver Detect Shows... | AJMC Early Stage Cancer [https://www.ajmc.com/view/liquid-biopsy-to-detect-early-stage-liver-cancer-shows-higher-sensitivity-specificity]
- 67. A Quantitative Framework to Study Potential Benefits and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8755582/]
- 68. Liquid biopsy in clinical practice: current evidence and ... [https://www.sciencedirect.com/science/article/pii/B9780443274961000105]
- 69. Pre-Analytical, Analytical, & Post-Analytical Phases of Lab ... [https://genemod.net/blog/pre-analytical-lab-testing-phase]
- 70. Pre-Analytical Requirements - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC2556574/]
- 71. Quantitative and Qualitative Analysis of Blood-based Liquid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8941682/]
- 72. False Positive ctDNA Results Do Not Explain Lack of Efficacy ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11371381/]
- 73. Clonal hematopoiesis in metastatic urothelial and renal cell ... [https://www.nature.com/articles/s41698-025-00965-y]
- 74. Q&A: Exploring the implications of CHIP on oncology ... [https://www.tempus.com/resources/content/articles/qa-exploring-the-implications-of-chip-on-oncology-patient-care/?srsltid=AfmBOoryxG6Yvg_qP6Kv5oB4_UZXo4g3gyO4LUj58Gp22aVnrdJvXO8l]
- 75. Research progress of CTC, ctDNA, and EVs in cancer liquid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10850354/]
- 76. Enhancing detection and monitoring of circulating tumor cells [https://www.sciencedirect.com/science/article/pii/S295019542500013X]
- 77. Use of ctDNA in early breast cancer: analytical validity and ... [https://www.nature.com/articles/s41523-024-00653-3]
- 78. Integrating Circulating Tumor DNA into Clinical Management ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12346868/]
- 79. Efficacy of liquid biopsy for genetic mutations determination in ... [https://bmccancer.biomedcentral.com/articles/10.1186/s12885-025-13786-w]
- 80. Artificial Intelligence-Enhanced Liquid Biopsy and Radiomics ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12524159/]
- 81. Artificial Intelligence for the Diagnosis and Management of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12587170/]
- 82. The AI revolution: how multimodal intelligence will reshape ... [https://www.nature.com/articles/s44387-025-00044-4]
- 83. Quantum AI creates a better liquid biopsy for cancer [https://pme.uchicago.edu/news/quantum-ai-creates-better-liquid-biopsy-cancer]
- 84. Data Analysis for Quantitative Data: Techniques and Tools ... [https://www.newhorizons.com/resources/blog/data-analysis-for-quantitative]
- 85. What is Quantitative Data Analysis? A Comprehensive Guide [https://airbyte.com/data-engineering-resources/what-is-quantitative-data-analysis]
- 86. Your Data Visualization Color Guide: 7 Best Practices | Sigma [https://www.sigmacomputing.com/blog/7-best-practices-for-using-color-in-data-visualizations]
- 87. Limit of Blank, Limit of Detection and Limit of Quantitation [https://pmc.ncbi.nlm.nih.gov/articles/PMC2556583/]
- 88. Approaches to Assay Characterization and Validation: Part 1 [https://www.quanterix.com/approaches-to-assay-characterization-and-validation-part-1-limit-of-detection-and-lower-limit-of-quantitation/]
- 89. Accuracy and precision [https://en.wikipedia.org/wiki/Accuracy_and_precision]
- 90. Practices of Science: Precision vs. Accuracy [https://manoa.hawaii.edu/exploringourfluidearth/physical/world-ocean/map-distortion/practices-science-precision-vs-accuracy]
- 91. - Wikipedia Reproducibility [https://en.wikipedia.org/wiki/Reproducibility]
- 92. of Scientific Results (Stanford Encyclopedia of...) Reproducibility [https://plato.stanford.edu/entries/scientific-reproducibility/]
- 93. The Limit of Detection [https://www.chromatographyonline.com/view/limit-detection]
- 94. Liquid biopsy- A pivotal test to help navigate clinical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12409318/]
- 95. Validation of a liquid biopsy assay with increased sensitivity for clinical comprehensive genomic profiling - ScienceDirect [https://www.sciencedirect.com/science/article/pii/S2950195425000384]
- 96. Liquid Biopsy | September Round-Up 2025 [https://www.decibio.com/insights/liquid-biopsy-september-round-up-2025]
- 97. Validation of a liquid biopsy assay with increased sensitivity for clinical comprehensive genomic profiling - PubMed [https://pubmed.ncbi.nlm.nih.gov/40980342/]
- 98. Concordance of Genomic Profiles in Matched Tissue and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9597027/]
- 99. Comparative analysis of genomic profiles between tissue ... [https://www.sciencedirect.com/science/article/abs/pii/S0169500223008206]
- 100. DAB Chromogen/Substrate Kit (High Contrast) [https://www.scytek.com/products/209.83-ACT500-DAB-Chromogen-Substrate-Kit-(High-Contrast).asp]
- 101. Chromogenic Western Blotting Substrates [https://www.thermofisher.com/tr/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/chromogenic-western-blotting-substrates.html]
- 102. Chromogenic Substrates Overview [https://www.goldbio.com/blogs/articles/chromogenic-substrates-overview?srsltid=AfmBOooeDyHjn6ZvhyXD3uL9sqk5UrZEmyM-OVe6zNigl3GUblOTxjTr]
- 103. Circular RNA-based liquid : a promising approach for... biopsy [https://www.oaepublish.com/articles/cdr.2025.142]
- 104. Circulating Tumor DNA as a Prognostic and Predictive ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12564818/]
- 105. Circulating tumor DNA as prognostic marker in patients ... [https://www.sciencedirect.com/science/article/pii/S0305737225001215]
- 106. DYNAMIC Study 5-Year Outcomes Provide OS and ctDNA ... [https://www.gioncologynow.com/post/dynamic-study-5-year-outcomes-provide-os-and-ctdna-insight]
- 107. Circulating tumor DNA as a biomarker of prognosis ... [https://www.sciencedirect.com/science/article/pii/S2667005424001169]
- 108. Correlation between circulating tumor DNA quantity ... [https://www.nature.com/articles/s41598-025-13160-7]